Pyroptosis in host defence against bacterial infection
- PMID: 35801644
- PMCID: PMC10623139
- DOI: 10.1242/dmm.049414
Pyroptosis in host defence against bacterial infection
Abstract
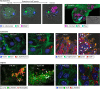
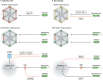
Pyroptosis, a regulated form of pro-inflammatory cell death, is characterised by cell lysis and by the release of cytokines, damage- and pathogen-associated molecular patterns. It plays an important role during bacterial infection, where it can promote an inflammatory response and eliminate the replicative niche of intracellular pathogens. Recent work, using a variety of bacterial pathogens, has illuminated the versatility of pyroptosis, revealing unexpected and important concepts underlying host defence. In this Review, we overview the molecular mechanisms underlying pyroptosis and discuss their role in host defence, from the single cell to the whole organism. We focus on recent studies using three cellular microbiology paradigms - Mycobacterium tuberculosis, Salmonella Typhimurium and Shigella flexneri - that have transformed the field of pyroptosis. We compare insights discovered in tissue culture, zebrafish and mouse models, highlighting the advantages and disadvantages of using these complementary infection models to investigate pyroptosis and for modelling human infection. Moving forward, we propose that in-depth knowledge of pyroptosis obtained from complementary infection models can better inform future studies using higher vertebrates, including humans, and help develop innovative host-directed therapies to combat bacterial infection.
Keywords: Salmonella; Shigella; Bacterial infection; Cell death; Cell-autonomous immunity; Host-pathogen interaction; Mycobacteria; Pyroptosis.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
References
-
- Bauernfeind, F., Horvath, G., Stutz, A., Alnemri, E. S., Speert, D., Fernandes-alnemri, T., Wu, J., Brian, G., Fitzgerald, K. A., Hornung, V.et al. (2010). NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787-791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

