Benefits of esmolol in adults with sepsis and septic shock: An updated meta-analysis of randomized controlled trials
- PMID: 35801730
- PMCID: PMC9259117
- DOI: 10.1097/MD.0000000000029820
Benefits of esmolol in adults with sepsis and septic shock: An updated meta-analysis of randomized controlled trials
Abstract
Background: Sepsis affects millions of patients annually, resulting in substantial health and economic burdens globally. The role of esmolol potentially plays in the treatment of sepsis and septic shock in adult patients remains controversial.
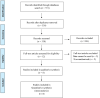
Methods: We undertook a systematic search of PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases from their inception to May 12, 2022, for randomized controlled trials that evaluated the efficacy of esmolol for sepsis and septic shock. A random-effects meta-analysis was performed. Two investigators independently screened articles, extracted data, and assessed the quality of included studies.
Results: Eight studies from 7 randomized controlled trials were included in our meta-analysis of 503 patients with sepsis and/or septic shock. Compared with standard treatment, esmolol significantly decreased 28-day mortality (risk ratio 0.68, 95% confidence interval [CI] 0.52-0.88; P = .004), heart rate (standardized mean difference [SMD] -1.83, 95% CI -2.95 to -0.70, P = .001), tumor necrosis factor-a (SMD -0.48, 95% CI -0.94 to -0.02, P = .04), and the troponin I level (SMD -0.59, 95% CI -1.02 to -0.16, P = .008) 24 hours after treatment. No significant effect was found in terms of length of intensive care unit stay; mean arterial pressure, lactic acid, central venous pressure, or central venous oxygen saturation, interleukin 6, or white blood cell levels; stroke volume index; or the PaO2/FiO2 ratio.
Conclusions: Esmolol treatment may be safe and effective in decreasing 28-day mortality, controlling heart rate, and providing cardioprotective function, but has no effect on lung injury in patients with sepsis or septic shock after early fluid resuscitation. Improvement in cardiac function may be related to changes in serum inflammatory mediators. No significant adverse effects on tissue perfusion and oxygen utilization were observed.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures



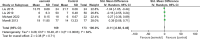







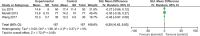
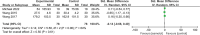


Similar articles
-
The role of esmolol in sepsis: a meta-analysis based on randomized controlled trials.BMC Anesthesiol. 2024 Sep 12;24(1):326. doi: 10.1186/s12871-024-02714-3. BMC Anesthesiol. 2024. PMID: 39266951 Free PMC article.
-
Prognosis of β-adrenergic blockade therapy on septic shock and sepsis: A systematic review and meta-analysis of randomized controlled studies.Cytokine. 2020 Feb;126:154916. doi: 10.1016/j.cyto.2019.154916. Epub 2019 Nov 19. Cytokine. 2020. PMID: 31756644
-
[Esmolol improves clinical outcome and tissue oxygen metabolism in patients with septic shock through controlling heart rate].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015 Sep;27(9):759-63. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015. PMID: 26955704 Clinical Trial. Chinese.
-
VERY EARLY USE OF ESMOLOL IN HYPERKINETIC SEPTIC SHOCK PATIENTS WITH PERSISTENT TACHYCARDIA: A RANDOMIZED CONTROLLED PILOT STUDY.Shock. 2025 Jun 1;63(6):870-877. doi: 10.1097/SHK.0000000000002576. Epub 2025 Mar 3. Shock. 2025. PMID: 40101954 Clinical Trial.
-
Effect of Ultrashort-Acting β-Blockers on Mortality in Patients With Sepsis With Persistent Tachycardia Despite Initial Resuscitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Chest. 2021 Jun;159(6):2289-2300. doi: 10.1016/j.chest.2021.01.009. Epub 2021 Jan 9. Chest. 2021. PMID: 33434497
Cited by
-
The concomitant use of ultra short beta-blockers with vasopressors and inotropes in critically ill patients with septic shock: A systematic review and meta-analysis of randomized controlled trials.Saudi Pharm J. 2024 Jun;32(6):102094. doi: 10.1016/j.jsps.2024.102094. Epub 2024 May 11. Saudi Pharm J. 2024. PMID: 38812943 Free PMC article.
-
Efficacy and Safety of Landiolol in the Treatment of Tachycardia in Patients With Sepsis and Septic Shock: A Systematic Review and Meta-Analysis.Cureus. 2025 Jul 15;17(7):e88004. doi: 10.7759/cureus.88004. eCollection 2025 Jul. Cureus. 2025. PMID: 40821221 Free PMC article. Review.
-
Esmolol's Role in Hemodynamic Management During Pheochromocytoma Surgery: A Comprehensive Review.Cureus. 2024 Jun 6;16(6):e61786. doi: 10.7759/cureus.61786. eCollection 2024 Jun. Cureus. 2024. PMID: 38975526 Free PMC article. Review.
-
Clinical Effect of Norepinephrine Combined with Esmolol Treatment in Patients with Septic Shock and Its Impact on Prognosis.Int J Gen Med. 2024 Sep 23;17:4325-4333. doi: 10.2147/IJGM.S477593. eCollection 2024. Int J Gen Med. 2024. PMID: 39346634 Free PMC article.
-
The influence of metoprolol in patients with sepsis-induced cardiomyopathy: A retrospective study.Saudi Med J. 2023 Oct;44(10):1030-1036. doi: 10.15537/smj.2023.44.10.20230149. Saudi Med J. 2023. PMID: 37777259 Free PMC article.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. - PubMed
-
- Dombrovskiy VY, Martin AA, Sunderram J, et al. . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

