COVID-19 therapeutics and outcomes among solid organ transplant recipients during the Omicron BA.1 era
- PMID: 35801839
- PMCID: PMC9349644
- DOI: 10.1111/ajt.17140
COVID-19 therapeutics and outcomes among solid organ transplant recipients during the Omicron BA.1 era
Abstract
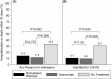
Treatment outcomes associated with the use of novel COVID-19 therapeutics in solid organ transplant recipients (SOTR) are not well described in the literature. The objective of this analysis was to characterize 30-day hospitalization and other key secondary endpoints experienced by outpatient SOTR with mild-moderate COVID-19 treated with nirmatrelvir/ritonavir (NR), sotrovimab, or no SARS-CoV-2 specific treatment. This IRB-approved, retrospective study included 154 SOTR with a documented positive SARS-CoV-2 infection between December 16, 2021 and January 19, 2022 (a predominant Omicron BA.1 period in New York City). Patients who received NR (N = 28) or sotrovimab (N = 51) experienced a lower rate of 30-day hospitalization or death as compared to those who received no specific treatment (N = 75) (p = .009). A total of three deaths occurred, all among patients who initially received no specific treatment prior to hospitalization. These results suggest a role for SARS-CoV-2 specific agents in the treatment of SOTR with COVID-19, and that there does not appear to be any difference in effectiveness when comparing NR versus sotrovimab.
© 2022 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
Similar articles
-
Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge.Am J Transplant. 2022 Oct;22(10):2458-2463. doi: 10.1111/ajt.17098. Epub 2022 May 30. Am J Transplant. 2022. PMID: 35583664 Free PMC article.
-
Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023.Am J Transplant. 2024 Jul;24(7):1303-1316. doi: 10.1016/j.ajt.2024.03.011. Epub 2024 Mar 17. Am J Transplant. 2024. PMID: 38499087
-
Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch.Transpl Infect Dis. 2022 Aug;24(4):e13901. doi: 10.1111/tid.13901. Epub 2022 Jul 25. Transpl Infect Dis. 2022. PMID: 35848574 Free PMC article.
-
Sotrovimab therapy in solid organ transplant recipients with mild to moderate COVID-19: a systematic review and meta-analysis.Immunopharmacol Immunotoxicol. 2023 Dec;45(4):402-408. doi: 10.1080/08923973.2022.2160733. Epub 2022 Dec 27. Immunopharmacol Immunotoxicol. 2023. PMID: 36537311
-
Antispike monoclonal antibodies for prevention and treatment of coronavirus disease-2019 in solid organ transplant recipients.Curr Opin Organ Transplant. 2022 Aug 1;27(4):269-276. doi: 10.1097/MOT.0000000000000981. Curr Opin Organ Transplant. 2022. PMID: 36354253 Review.
Cited by
-
COVID-19 in solid organ transplant recipients after 2 years of pandemic: Outcome and impact of antiviral treatments in a single-center study.Front Transplant. 2023 Mar 16;2:1095225. doi: 10.3389/frtra.2023.1095225. eCollection 2023. Front Transplant. 2023. PMID: 38993895 Free PMC article.
-
T follicular helper cell responses to SARS-CoV-2 vaccination among healthy and immunocompromised adults.Immunol Cell Biol. 2023 Jul;101(6):504-513. doi: 10.1111/imcb.12635. Epub 2023 Mar 23. Immunol Cell Biol. 2023. PMID: 36825370 Free PMC article. Review.
-
Monoclonal Antibodies in Prevention and Early Treatment of COVID-19 in Lung Transplant Recipients: A Systematic Review and Perspective on the Role of Monoclonal Antibodies in the Future.Transpl Int. 2025 Feb 10;38:13800. doi: 10.3389/ti.2025.13800. eCollection 2025. Transpl Int. 2025. PMID: 39995815 Free PMC article.
-
Comparison of COVID-19 Hospitalization and Death Between Solid Organ Transplant Recipients and the General Population in Canada, 2020-2022.Transplant Direct. 2024 Jun 26;10(7):e1670. doi: 10.1097/TXD.0000000000001670. eCollection 2024 Jul. Transplant Direct. 2024. PMID: 38953040 Free PMC article.
-
Clinical Outcomes of SARS-CoV-2 Breakthrough Infections in Liver Transplant Recipients during the Omicron Wave.Viruses. 2023 Jan 20;15(2):297. doi: 10.3390/v15020297. Viruses. 2023. PMID: 36851510 Free PMC article.
References
-
- Trapani S, Masiero L, Puoti F, et al. the Italian Network of Regional Transplant Coordinating Centers Collaborating Group, Italian Surveillance System of Covid-19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN-SITO Study Group Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2021;21(7):2509–2521. doi: 10.1111/ajt.16428. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous