Short LNA-modified oligonucleotide probes as efficient disruptors of DNA G-quadruplexes
- PMID: 35801856
- PMCID: PMC9303293
- DOI: 10.1093/nar/gkac569
Short LNA-modified oligonucleotide probes as efficient disruptors of DNA G-quadruplexes
Abstract
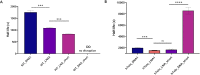
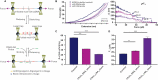
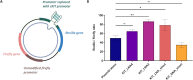
G-quadruplexes (G4s) are well known non-canonical DNA secondary structures that can form in human cells. Most of the tools available to investigate G4-biology rely on small molecule ligands that stabilise these structures. However, the development of probes that disrupt G4s is equally important to study their biology. In this study, we investigated the disruption of G4s using Locked Nucleic Acids (LNA) as invader probes. We demonstrated that strategic positioning of LNA-modifications within short oligonucleotides (10 nts.) can significantly accelerate the rate of G4-disruption. Single-molecule experiments revealed that short LNA-probes can promote disruption of G4s with mechanical stability sufficient to stall polymerases. We corroborated this using a single-step extension assay, revealing that short LNA-probes can relieve replication dependent polymerase-stalling at G4 sites. We further demonstrated the potential of such LNA-based probes to study G4-biology in cells. By using a dual-luciferase assay, we found that short LNA probes can enhance the expression of c-KIT to levels similar to those observed when the c-KIT promoter is mutated to prevent the formation of the c-KIT1 G4. Collectively, our data suggest a potential use of rationally designed LNA-modified oligonucleotides as an accessible chemical-biology tool for disrupting individual G4s and interrogating their biological functions in cells.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
The Emerging Roles of Multimolecular G-Quadruplexes in Transcriptional Regulation and Chromatin Organization.Acc Chem Res. 2024 Dec 3;57(23):3397-3406. doi: 10.1021/acs.accounts.4c00574. Epub 2024 Nov 18. Acc Chem Res. 2024. PMID: 39555660 Free PMC article.
-
Effect of LNA- and OMeN-modified oligonucleotide probes on the stability and discrimination of mismatched base pairs of duplexes.J Biosci. 2012 Jun;37(2):233-41. doi: 10.1007/s12038-012-9196-4. J Biosci. 2012. PMID: 22581329
-
Recognition of mixed-sequence double-stranded DNA regions using chimeric Invader/LNA probes.Org Biomol Chem. 2025 Jan 15;23(3):619-628. doi: 10.1039/d4ob01403k. Org Biomol Chem. 2025. PMID: 39412680 Free PMC article.
-
Advances in G-quadruplexes-based fluorescent imaging.Biopolymers. 2022 Dec;113(12):e23528. doi: 10.1002/bip.23528. Epub 2022 Nov 29. Biopolymers. 2022. PMID: 36444749 Review.
-
Specific Recognition of Promoter G-Quadruplex DNAs by Small Molecule Ligands and Light-up Probes.ACS Chem Biol. 2019 Oct 18;14(10):2102-2114. doi: 10.1021/acschembio.9b00475. Epub 2019 Oct 4. ACS Chem Biol. 2019. PMID: 31532996 Review.
Cited by
-
Structural Unfolding of G-Quadruplexes: From Small Molecules to Antisense Strategies.Molecules. 2024 Jul 25;29(15):3488. doi: 10.3390/molecules29153488. Molecules. 2024. PMID: 39124893 Free PMC article. Review.
-
Long-range DNA interactions: inter-molecular G-quadruplexes and their potential biological relevance.Chem Commun (Camb). 2022 Nov 17;58(92):12753-12762. doi: 10.1039/d2cc04872h. Chem Commun (Camb). 2022. PMID: 36281554 Free PMC article. Review.
-
Genomic Analysis of Non-B Nucleic Acids Structures in SARS-CoV-2: Potential Key Roles for These Structures in Mutability, Translation, and Replication?Genes (Basel). 2023 Jan 6;14(1):157. doi: 10.3390/genes14010157. Genes (Basel). 2023. PMID: 36672896 Free PMC article.
-
Artificially inserted strong promoter containing multiple G-quadruplexes induces long-range chromatin modification.Elife. 2024 Aug 19;13:RP96216. doi: 10.7554/eLife.96216. Elife. 2024. PMID: 39158543 Free PMC article.
-
Roles of G4-DNA and G4-RNA in Class Switch Recombination and Additional Regulations in B-Lymphocytes.Molecules. 2023 Jan 24;28(3):1159. doi: 10.3390/molecules28031159. Molecules. 2023. PMID: 36770824 Free PMC article. Review.
References
-
- Raguseo F., Chowdhury S., Minard A., Di Antonio M.. Chemical-biology approaches to probe DNA and RNA G-quadruplex structures in the genome. Chem. Commun. 2020; 56:1317–1324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

