Structural and functional characterization of DdrC, a novel DNA damage-induced nucleoid associated protein involved in DNA compaction
- PMID: 35801857
- PMCID: PMC9303277
- DOI: 10.1093/nar/gkac563
Structural and functional characterization of DdrC, a novel DNA damage-induced nucleoid associated protein involved in DNA compaction
Erratum in
-
Correction to 'Structural and functional characterization of DdrC, a novel DNA damage-induced nucleoid associated protein involved in DNA compaction'.Nucleic Acids Res. 2023 Feb 28;51(4):1997. doi: 10.1093/nar/gkad041. Nucleic Acids Res. 2023. PMID: 36651284 Free PMC article. No abstract available.
Abstract
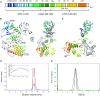
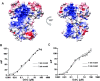
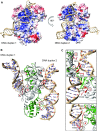
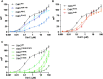
Deinococcus radiodurans is a spherical bacterium well-known for its outstanding resistance to DNA-damaging agents. Exposure to such agents leads to drastic changes in the transcriptome of D. radiodurans. In particular, four Deinococcus-specific genes, known as DNA Damage Response genes, are strongly up-regulated and have been shown to contribute to the resistance phenotype of D. radiodurans. One of these, DdrC, is expressed shortly after exposure to γ-radiation and is rapidly recruited to the nucleoid. In vitro, DdrC has been shown to compact circular DNA, circularize linear DNA, anneal complementary DNA strands and protect DNA from nucleases. To shed light on the possible functions of DdrC in D. radiodurans, we determined the crystal structure of the domain-swapped DdrC dimer at a resolution of 2.5 Å and further characterized its DNA binding and compaction properties. Notably, we show that DdrC bears two asymmetric DNA binding sites located on either side of the dimer and can modulate the topology and level of compaction of circular DNA. These findings suggest that DdrC may be a DNA damage-induced nucleoid-associated protein that enhances nucleoid compaction to limit the dispersion of the fragmented genome and facilitate DNA repair after exposure to severe DNA damaging conditions.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
DdrC, a unique DNA repair factor from D. radiodurans, senses and stabilizes DNA breaks through a novel lesion-recognition mechanism.Nucleic Acids Res. 2024 Aug 27;52(15):9282-9302. doi: 10.1093/nar/gkae635. Nucleic Acids Res. 2024. PMID: 39036966 Free PMC article.
-
In vivo and in vitro characterization of DdrC, a DNA damage response protein in Deinococcus radiodurans bacterium.PLoS One. 2017 May 18;12(5):e0177751. doi: 10.1371/journal.pone.0177751. eCollection 2017. PLoS One. 2017. PMID: 28542368 Free PMC article.
-
The essential histone-like protein HU plays a major role in Deinococcus radiodurans nucleoid compaction.Mol Microbiol. 2009 Jul;73(2):240-52. doi: 10.1111/j.1365-2958.2009.06766.x. Epub 2009 Jun 28. Mol Microbiol. 2009. PMID: 19570109
-
Gene regulation for the extreme resistance to ionizing radiation of Deinococcus radiodurans.Gene. 2019 Oct 5;715:144008. doi: 10.1016/j.gene.2019.144008. Epub 2019 Jul 27. Gene. 2019. PMID: 31362038 Review.
-
Oxidative stress resistance in Deinococcus radiodurans.Microbiol Mol Biol Rev. 2011 Mar;75(1):133-91. doi: 10.1128/MMBR.00015-10. Microbiol Mol Biol Rev. 2011. PMID: 21372322 Free PMC article. Review.
Cited by
-
Stress-induced nucleoid remodeling in Deinococcus radiodurans is associated with major changes in Heat Unstable (HU) protein dynamics.Nucleic Acids Res. 2024 Jun 24;52(11):6406-6423. doi: 10.1093/nar/gkae379. Nucleic Acids Res. 2024. PMID: 38742631 Free PMC article.
-
Surviving the storm: exploring the role of natural transformation in nutrition and DNA repair of stressed Deinococcus radiodurans.Appl Environ Microbiol. 2025 Jan 31;91(1):e0137124. doi: 10.1128/aem.01371-24. Epub 2024 Dec 9. Appl Environ Microbiol. 2025. PMID: 39651863 Free PMC article.
-
New Insights into Radio-Resistance Mechanism Revealed by (Phospho)Proteome Analysis of Deinococcus Radiodurans after Heavy Ion Irradiation.Int J Mol Sci. 2023 Oct 1;24(19):14817. doi: 10.3390/ijms241914817. Int J Mol Sci. 2023. PMID: 37834265 Free PMC article.
-
DdrC, a unique DNA repair factor from D. radiodurans, senses and stabilizes DNA breaks through a novel lesion-recognition mechanism.Nucleic Acids Res. 2024 Aug 27;52(15):9282-9302. doi: 10.1093/nar/gkae635. Nucleic Acids Res. 2024. PMID: 39036966 Free PMC article.
-
To split or not to split: CASP15 targets and their processing into tertiary structure evaluation units.Proteins. 2023 Dec;91(12):1558-1570. doi: 10.1002/prot.26533. Epub 2023 May 31. Proteins. 2023. PMID: 37254889 Free PMC article.
References
-
- Battista J.R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997; 51:203–224. - PubMed
-
- Daly M.J. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004; 306:1025–1028. - PubMed
-
- Cox M.M., Battista J.R.. Deinococcus radiodurans - the consummate survivor. Nat. Rev. Microbiol. 2005; 3:882–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

