NAT10-mediated N4-acetylcytidine modification is required for meiosis entry and progression in male germ cells
- PMID: 35801907
- PMCID: PMC9638909
- DOI: 10.1093/nar/gkac594
NAT10-mediated N4-acetylcytidine modification is required for meiosis entry and progression in male germ cells
Abstract
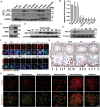
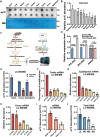
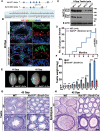
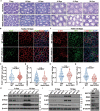
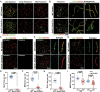
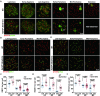
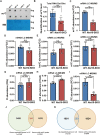
Post-transcriptional RNA modifications critically regulate various biological processes. N4-acetylcytidine (ac4C) is an epi-transcriptome, which is highly conserved in all species. However, the in vivo physiological functions and regulatory mechanisms of ac4C remain poorly understood, particularly in mammals. In this study, we demonstrate that the only known ac4C writer, N-acetyltransferase 10 (NAT10), plays an essential role in male reproduction. We identified the occurrence of ac4C in the mRNAs of mouse tissues and showed that ac4C undergoes dynamic changes during spermatogenesis. Germ cell-specific ablation of Nat10 severely inhibits meiotic entry and leads to defects in homologous chromosome synapsis, meiotic recombination and repair of DNA double-strand breaks during meiosis. Transcriptomic profiling revealed dysregulation of functional genes in meiotic prophase I after Nat10 deletion. These findings highlight the crucial physiological functions of ac4C modifications in male spermatogenesis and expand our understanding of its role in the regulation of specific physiological processes in vivo.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

