Risankizumab improved health-related quality of life, fatigue, pain and work productivity in psoriatic arthritis: results of KEEPsAKE 1
- PMID: 35801915
- PMCID: PMC9891435
- DOI: 10.1093/rheumatology/keac342
Risankizumab improved health-related quality of life, fatigue, pain and work productivity in psoriatic arthritis: results of KEEPsAKE 1
Abstract
Objectives: PsA is a heterogeneous disease that impacts many aspects of social and mental life, including quality of life. Risankizumab, an antagonist specific for IL-23, is currently under investigation for the treatment of adults with active PsA. This study evaluated the impact of risankizumab vs placebo on health-related quality of life (HRQoL) and other patient-reported outcomes (PROs) among patients with active PsA and inadequate response or intolerance to conventional synthetic DMARD (csDMARD-IR) in the KEEPsAKE 1 trial.
Methods: Adult patients with active PsA (n = 964) were randomized (1:1) to receive risankizumab 150 mg or placebo. PROs assessed included the 36-Item Short-Form Health Survey (SF-36, v2), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), EuroQoL-5 Dimension-5 Level (EQ-5D-5L), Patient's Assessment of Pain, Patient's Global Assessment (PtGA) of Disease Activity, and Work Productivity and Activity Impairment-PsA (WPAI-PsA) questionnaire. Least squares (LS) mean change from baseline at week 24 was compared between risankizumab and placebo.
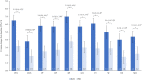
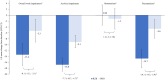
Results: At week 24, differences between groups were observed using LS mean changes from baseline in SF-36 physical component summary and mental component summary; FACIT-Fatigue; EQ-5D-5L; Patient's Assessment of Pain; PtGA; all eight SF-36 domains (all nominal P < 0.001); and the WPAI-PsA domains of impairment while working (presenteeism), overall work impairment and activity impairment (all nominal P < 0.01).
Conclusion: Risankizumab treatment resulted in greater improvements in HRQoL, fatigue, pain and work productivity in patients with active PsA who have csDMARD-IR, when compared with placebo.
Trial registration: ClinicalTrials.gov, https://clinicaltrials.gov, NCT03675308.
Keywords: DMARDs; biological therapies; outcome measures; patient attitude to health; quality of life; spondyloarthropathies (including psoriatic arthritis).
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Duarte GV, Faillace C, de Carvalho JF.. Psoriatic arthritis [review] . Best Pract Res Clin Rheumatol 2012;26:147–56. - PubMed
-
- Gladman DD, Chandran V.. Observational cohort studies: lessons learnt from the University of Toronto psoriatic arthritis program [review] . Rheumatology 2011;50:25–31. - PubMed
-
- Kristensen LE, Englund M, Neovius M. et al. Long-term work disability in patients with psoriatic arthritis treated with anti-tumour necrosis factor: a population-based regional Swedish cohort study. Ann Rheum Dis 2013;72:1675–9. - PubMed
-
- Kristensen LE, Jorgensen TS, Christensen R. et al. Societal costs and patients' experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis 2017;76:1495–501. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

