A host-vector toolbox for improved secretory protein overproduction in Bacillus subtilis
- PMID: 35802157
- PMCID: PMC9329435
- DOI: 10.1007/s00253-022-12062-2
A host-vector toolbox for improved secretory protein overproduction in Bacillus subtilis
Abstract
Target proteins in biotechnological applications are highly diverse. Therefore, versatile flexible expression systems for their functional overproduction are required. In order to find the right heterologous gene expression strategy, suitable host-vector systems, which combine different genetic circuits, are useful. In this study, we designed a novel Bacillus subtilis expression toolbox, which allows the overproduction and secretion of potentially toxic enzymes. This toolbox comprises a set of 60 expression vectors, which combine two promoter variants, four strong secretion signals, a translation-enhancing downstream box, and three plasmid backbones. This B. subtilis toolbox is based on a tailor-made, clean deletion mutant strain, which is protease and sporulation deficient and exhibits reduced autolysis and secondary metabolism. The appropriateness of this alternative expression platform was tested for the overproduction of two difficult-to-produce eukaryotic model proteins. These included the sulfhydryl oxidase Sox from Saccharomyces cerevisiae, which forms reactive hydrogen peroxide and undesired cross-linking of functional proteins, and the human interleukin-1β, a pro-inflammatory cytokine. For the best performing Sox and interleukin, overproducing and secreting variants of these new B. subtilis toolbox fermentation strategies were developed and tested. This study demonstrates the suitability of the prokaryotic B. subtilis host-vector system for the extracellular production of two eukaryotic proteins with biotechnological relevance. KEY POINTS: • Construction of a versatile Bacillus subtilis gene expression toolbox. • Verification of the toolbox by the secretory overproduction of two difficult-to-express proteins. • Fermentation strategy for an acetoin-controlled overproduction of heterologous proteins.
Keywords: Bacillus subtilis; Expression system; Fed batch; Human interleukin-1β; Overproduction; Protease deficiency; Protein secretion; Yeast sulfhydryl oxidase.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
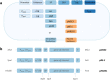

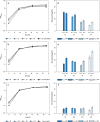



References
-
- Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J Mol Biol. 2006;362:393–402. doi: 10.1016/j.jmb.2006.07.034. - DOI - PubMed
-
- Emond E, Lavallée R, Drolet G, Moineau S, LaPointe G. Molecular characterization of a theta replication plasmid and its use for development of a two-component food-grade cloning system for Lactococcus lactis. Appl Environ Microbiol. 2001;67:1700–1709. doi: 10.1128/AEM.67.4.1700-1709.2001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

