Comparative Effectiveness of Diversion of Cerebrospinal Fluid for Children With Severe Traumatic Brain Injury
- PMID: 35802371
- PMCID: PMC9270700
- DOI: 10.1001/jamanetworkopen.2022.20969
Comparative Effectiveness of Diversion of Cerebrospinal Fluid for Children With Severe Traumatic Brain Injury
Erratum in
-
Errors in Abstract and Main Text Results.JAMA Netw Open. 2022 Aug 1;5(8):e2232865. doi: 10.1001/jamanetworkopen.2022.32865. JAMA Netw Open. 2022. PMID: 36044225 Free PMC article. No abstract available.
Abstract
Importance: Diversion of cerebrospinal fluid (CSF) has been used for decades as a treatment for children with severe traumatic brain injury (TBI) and is recommended by evidenced-based guidelines. However, these recommendations are based on limited studies.
Objective: To determine whether CSF diversion is associated with improved Glasgow Outcome Score-Extended for Pediatrics (GOS-EP) and decreased intracranial pressure (ICP) in children with severe TBI.
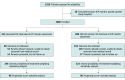
Design, setting, and participants: This observational comparative effectiveness study was performed at 51 clinical centers that routinely care for children with severe TBI in 8 countries (US, United Kingdom, Spain, the Netherlands, Australia, New Zealand, South Africa, and India) from February 2014 to September 2017, with follow-up at 6 months after injury (final follow-up, October 22, 2021). Children with severe TBI were included if they had Glasgow Coma Scale (GCS) scores of 8 or lower, had intracranial pressure (ICP) monitor placed on-site, and were aged younger than 18 years. Children were excluded if they were pregnant or an ICP monitor was not placed at the study site. Consecutive children were screened and enrolled, data regarding treatments were collected, and at discharge, consent was obtained for outcomes testing. Propensity matching for pretreatment characteristics was performed to develop matched pairs for primary analysis. Data analyses were completed on April 18, 2022.
Exposures: Clinical care followed local standards, including the use of CSF diversion (or not), with patients stratified at the time of ICP monitor placement (CSF group vs no CSF group).
Main outcomes and measures: The primary outcome was GOS-EP at 6 months, while ICP was considered as a secondary outcome. CSF vs no CSF was treated as an intention-to-treat analysis, and a sensitivity analysis was performed for children who received delayed CSF diversion.
Results: A total of 1000 children with TBI were enrolled, including 314 who received CSF diversion (mean [SD] age, 7.18 [5.45] years; 208 [66.2%] boys) and 686 who did not (mean [SD] age, 7.79 [5.33] years; 437 [63.7%] boys). The propensity-matched analysis included 98 pairs. In propensity score-matched analyses, there was no difference between groups in GOS-EP (median [IQR] difference, 0 [-3 to 1]; P = .08), but there was a decrease in overall ICP in the CSF group (mean [SD] difference, 3.97 [0.12] mm Hg; P < .001).
Conclusions and relevance: In this comparative effectiveness study, CSF diversion was not associated with improved outcome at 6 months after TBI, but a decrease in ICP was observed. Given the higher quality of evidence generated by this study, current evidence-based guidelines related to CSF diversion should be reconsidered.
Conflict of interest statement
Figures

Comment in
-
The Role of Ventriculostomy in Severe Traumatic Brain Injury in Children-to Drain or Not to Drain?JAMA Netw Open. 2022 Jul 1;5(7):e2220978. doi: 10.1001/jamanetworkopen.2022.20978. JAMA Netw Open. 2022. PMID: 35802377 No abstract available.
References
-
- Cushing H. The Third Circulation in Studies of Intracranial Physiology and Surgery. Oxford University Press; 1926.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

