Strong and reliable synaptic communication between pyramidal neurons in adult human cerebral cortex
- PMID: 35802476
- PMCID: PMC10016070
- DOI: 10.1093/cercor/bhac246
Strong and reliable synaptic communication between pyramidal neurons in adult human cerebral cortex
Abstract
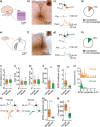
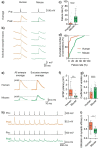
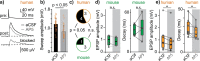
Synaptic transmission constitutes the primary mode of communication between neurons. It is extensively studied in rodent but not human neocortex. We characterized synaptic transmission between pyramidal neurons in layers 2 and 3 using neurosurgically resected human middle temporal gyrus (MTG, Brodmann area 21), which is part of the distributed language circuitry. We find that local connectivity is comparable with mouse layer 2/3 connections in the anatomical homologue (temporal association area), but synaptic connections in human are 3-fold stronger and more reliable (0% vs 25% failure rates, respectively). We developed a theoretical approach to quantify properties of spinous synapses showing that synaptic conductance and voltage change in human dendritic spines are 3-4-folds larger compared with mouse, leading to significant NMDA receptor activation in human unitary connections. This model prediction was validated experimentally by showing that NMDA receptor activation increases the amplitude and prolongs decay of unitary excitatory postsynaptic potentials in human but not in mouse connections. Since NMDA-dependent recurrent excitation facilitates persistent activity (supporting working memory), our data uncovers cortical microcircuit properties in human that may contribute to language processing in MTG.
Keywords: L2/L3; NMDA receptor; cortex; human brain; synaptic transmission.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures


References
-
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998:43:811–816. - PubMed
-
- Assaf Y, Bouznach A, Zomet O, Marom A, Yovel Y. Conservation of brain connectivity and wiring across the mammalian class. Nat Neurosci. 2020:23:805–808. - PubMed
-
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012:35:345–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

