Retinoic Acid Receptor Activation Reduces Metastatic Prostate Cancer Bone Lesions by Blocking the Endothelial-to-Osteoblast Transition
- PMID: 35802768
- PMCID: PMC9444986
- DOI: 10.1158/0008-5472.CAN-22-0170
Retinoic Acid Receptor Activation Reduces Metastatic Prostate Cancer Bone Lesions by Blocking the Endothelial-to-Osteoblast Transition
Abstract
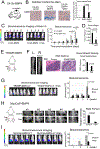
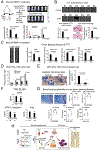
Metastatic prostate cancer in the bone induces bone-forming lesions that contribute to progression and therapy resistance. Prostate cancer-induced bone formation originates from endothelial cells (EC) that have undergone endothelial-to-osteoblast (EC-to-OSB) transition in response to tumor-secreted BMP4. Current strategies targeting prostate cancer-induced bone formation are lacking. Here, we show that activation of retinoic acid receptor (RAR) inhibits EC-to-OSB transition and reduces prostate cancer-induced bone formation. Treatment with palovarotene, an RARγ agonist being tested for heterotopic ossification in fibrodysplasia ossificans progressiva, inhibited EC-to-OSB transition and osteoblast mineralization in vitro and decreased tumor-induced bone formation and tumor growth in several osteogenic prostate cancer models, and similar effects were observed with the pan-RAR agonist all-trans-retinoic acid (ATRA). Knockdown of RARα, β, or γ isoforms in ECs blocked BMP4-induced EC-to-OSB transition and osteoblast mineralization, indicating a role for all three isoforms in prostate cancer-induced bone formation. Furthermore, treatment with palovarotene or ATRA reduced plasma Tenascin C, a factor secreted from EC-OSB cells, which may be used to monitor treatment response. Mechanistically, BMP4-activated pSmad1 formed a complex with RAR in the nucleus of ECs to activate EC-to-OSB transition. RAR activation by palovarotene or ATRA caused pSmad1 degradation by recruiting the E3-ubiquitin ligase Smad ubiquitination regulatory factor1 (Smurf1) to the nuclear pSmad1/RARγ complex, thus blocking EC-to-OSB transition. Collectively, these findings suggest that palovarotene can be repurposed to target prostate cancer-induced bone formation to improve clinical outcomes for patients with bone metastasis.
Significance: This study provides mechanistic insights into how RAR agonists suppress prostate cancer-induced bone formation and offers a rationale for developing RAR agonists for prostate cancer bone metastasis therapy. See related commentary by Bhowmick and Bhowmick, p. 2975.
©2022 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest
C. J. Logothetis reports receiving commercial research grants from Janssen, ORIC Pharmaceuticals, Novartis, Aragon Pharmaceuticals; and honoraria from Merck, Sharp & Dohme, Bayer, Amgen. No potential conflicts of interest are disclosed by the other authors.
Figures




Comment in
-
RARγ: The Bone of Contention for Endothelial Cells in Prostate Cancer Metastasis.Cancer Res. 2022 Sep 2;82(17):2975-2976. doi: 10.1158/0008-5472.CAN-22-2251. Cancer Res. 2022. PMID: 36052494
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

