Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis
- PMID: 35802849
- PMCID: PMC9671756
- DOI: 10.1200/JCO.22.01148
Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis
Abstract
Purpose: Photon involved-field radiotherapy (IFRT) is the standard-of-care radiotherapy for patients with leptomeningeal metastasis (LM) from solid tumors. We tested whether proton craniospinal irradiation (pCSI) encompassing the entire CNS would result in superior CNS progression-free survival (PFS) compared with IFRT.
Patients and methods: We conducted a randomized, phase II trial of pCSI versus IFRT in patients with non-small-cell lung cancer and breast cancers with LM. We enrolled patients with other solid tumors to an exploratory pCSI group. For the randomized groups, patients were assigned (2:1), stratified by histology and systemic disease status, to pCSI or IFRT. The primary end point was CNS PFS. Secondary end points included overall survival (OS) and treatment-related adverse events (TAEs).
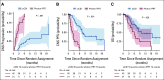
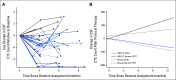
Results: Between April 16, 2020, and October 11, 2021, 42 and 21 patients were randomly assigned to pCSI and IFRT, respectively. At planned interim analysis, a significant benefit in CNS PFS was observed with pCSI (median 7.5 months; 95% CI, 6.6 months to not reached) compared with IFRT (2.3 months; 95% CI, 1.2 to 5.8 months; P < .001). We also observed OS benefit with pCSI (9.9 months; 95% CI, 7.5 months to not reached) versus IFRT (6.0 months; 95% CI, 3.9 months to not reached; P = .029). There was no difference in the rate of grade 3 and 4 TAEs (P = .19). In the exploratory pCSI group, 35 patients enrolled, the median CNS PFS was 5.8 months (95% CI, 4.4 to 9.1 months) and OS was 6.6 months (95% CI, 5.4 to 11 months).
Conclusion: Compared with photon IFRT, we found pCSI improved CNS PFS and OS for patients with non-small-cell lung cancer and breast cancer with LM with no increase in serious TAEs.
Trial registration: ClinicalTrials.gov NCT04343573.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
Comment in
-
Craniospinal irradiation improves leptomeningeal metastasis control.Nat Rev Clin Oncol. 2022 Sep;19(9):567. doi: 10.1038/s41571-022-00669-3. Nat Rev Clin Oncol. 2022. PMID: 35882997 No abstract available.
References
-
- Beauchesne P: Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 11:871-879, 2010 - PubMed
-
- Wasserstrom WR, Glass JP, Posner JB: Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer 49:759-772, 1982 - PubMed
-
- Kaplan JG, DeSouza TG, Farkash A, et al. : Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 9:225-229, 1990 - PubMed
-
- Chamberlain MC: Leptomeningeal metastasis. Curr Opin Oncol 22:627-635, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

