Oral Cancer Cells Release Vesicles that Cause Pain
- PMID: 35802912
- PMCID: PMC9474716
- DOI: 10.1002/adbi.202200073
Oral Cancer Cells Release Vesicles that Cause Pain
Abstract
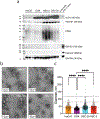
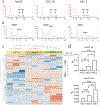
Oral cancer pain is attributed to the release from cancers of mediators that sensitize and activate sensory neurons. Intraplantar injection of conditioned media (CM) from human tongue cancer cell line HSC-3 or OSC-20 evokes nociceptive behavior. By contrast, CM from noncancer cell lines, DOK, and HaCaT are non-nociceptive. Pain mediators are carried by extracellular vesicles (EVs) released from cancer cells. Depletion of EVs from cancer cell line CM reverses mechanical allodynia and thermal hyperalgesia. CM from non-nociceptive cell lines become nociceptive when reconstituted with HSC-3 EVs. Two miRNAs (hsa-miR-21-5p and hsa-miR-221-3p) are identified that are present in increased abundance in EVs from HSC-3 and OSC-20 CM compared to HaCaT CM. The miRNA target genes suggest potential involvement in oral cancer pain of the toll like receptor 7 (TLR7) and 8 (TLR8) pathways, as well as signaling through interleukin 6 cytokine family signal transducer receptor (gp130, encoded by IL6ST) and colony stimulating factor receptor (G-CSFR, encoded by CSF3R), Janus kinase and signal transducer and activator of transcription 3 (JAK/STAT3). These studies confirm the recent discovery of the role of cancer EVs in pain and add to the repertoire of algesic and analgesic cancer pain mediators and pathways that contribute to oral cancer pain.
Keywords: extracellular vesicles; miR-21; miR-221; miRNA; oral cancer; oral cancer induced pain; pain.
© 2022 The Authors. Advanced Biology published by Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors state that they have no financial or commercial Conflicts of Interest.
Figures


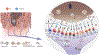
Similar articles
-
SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128.Viruses. 2023 Feb 24;15(3):622. doi: 10.3390/v15030622. Viruses. 2023. PMID: 36992331 Free PMC article.
-
Alterations in Abundance and Compartmentalization of miRNAs in Blood Plasma Extracellular Vesicles and Extracellular Condensates during HIV/SIV Infection and Its Modulation by Antiretroviral Therapy (ART) and Delta-9-Tetrahydrocannabinol (Δ9-THC).Viruses. 2023 Feb 24;15(3):623. doi: 10.3390/v15030623. Viruses. 2023. PMID: 36992332 Free PMC article.
-
Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma.BMC Cancer. 2018 Apr 18;18(1):439. doi: 10.1186/s12885-018-4364-z. BMC Cancer. 2018. PMID: 29669525 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
A pilot study to improve pain phenotyping in head and neck cancer patients.Front Pain Res (Lausanne). 2023 May 11;4:1146667. doi: 10.3389/fpain.2023.1146667. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37251594 Free PMC article.
-
miRNA packaging into small extracellular vesicles and implications in pain.Pain Rep. 2024 Oct 23;9(6):e1198. doi: 10.1097/PR9.0000000000001198. eCollection 2024 Dec. Pain Rep. 2024. PMID: 39450410 Free PMC article. Review.
-
Exosome-associated lysophosphatidic acid signaling contributes to cancer pain.Pain. 2023 Dec 1;164(12):2684-2695. doi: 10.1097/j.pain.0000000000002967. Epub 2023 Jun 6. Pain. 2023. PMID: 37278638 Free PMC article.
-
Oral Cancer Pain Includes Thermal Allodynia That May Be Attenuated by Chronic Alcohol Consumption.Pharmaceuticals (Basel). 2023 Mar 31;16(4):518. doi: 10.3390/ph16040518. Pharmaceuticals (Basel). 2023. PMID: 37111275 Free PMC article.
-
Cancer neuroscience in head and neck: interactions, modulation, and therapeutic strategies.Mol Cancer. 2025 Mar 31;24(1):101. doi: 10.1186/s12943-025-02299-6. Mol Cancer. 2025. PMID: 40165230 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

