miRNA induced 3D bioprinted-heterotypic osteochondral interface
- PMID: 35803212
- PMCID: PMC9588307
- DOI: 10.1088/1758-5090/ac7fbb
miRNA induced 3D bioprinted-heterotypic osteochondral interface
Abstract
The engineering of osteochondral interfaces remains a challenge. MicroRNAs (miRs) have emerged as significant tools to regulate the differentiation and proliferation of osteogenic and chondrogenic formation in the human musculoskeletal system. Here, we describe a novel approach to osteochondral reconstruction based on the three-dimensional (3D) bioprinting of miR-transfected adipose-derived stem cell (ADSC) spheroids to produce a heterotypic interface that addresses the intrinsic limitations of the traditional approach to inducing zonal differentiation via the use of diffusible cytokines. We evaluated the delivery of miR-148b for osteogenic differentiation and the codelivery of miR-140 and miR-21 for the chondrogenic differentiation of ADSC spheroids. Our results demonstrated that miR-transfected ADSC spheroids exhibited upregulated expression of osteogenic and chondrogenic differentiation related gene and protein markers, and enhanced mineralization and cell proliferation compared to spheroids differentiated using a commercially-available differentiation medium. Upon confirmation of the osteogenic and chondrogenic potential of miR-transfected ADSC spheroids, using aspiration-assisted bioprinting, these spheroids were 3D bioprinted into a dual-layer heterotypic osteochondral interface with a stratified arrangement of distinct osteogenic and chondrogenic zones. The proposed approach holds great promise for the biofabrication of stratified tissues, not only for the osteochondral interfaces presented in this work, but also for other composite tissues and tissue interfaces, such as, but not limited to, the bone-tendon-muscle interface and craniofacial tissues.
Keywords: bioprinting; bone; cartilage; miRNA; spheroids; stem cells.
© 2022 IOP Publishing Ltd.
Conflict of interest statement
COMPETING INTERESTS
I.T.O. has an equity stake in Biolife4D and is a member of the scientific advisory board for Biolife4D and Brinter. Other authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures






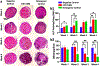


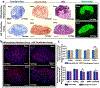
Similar articles
-
Aspiration-assisted bioprinting of the osteochondral interface.Sci Rep. 2020 Aug 4;10(1):13148. doi: 10.1038/s41598-020-69960-6. Sci Rep. 2020. PMID: 32753630 Free PMC article.
-
Scaffold-free bioprinted osteogenic and chondrogenic systems to model osteochondral physiology.Biomed Mater. 2019 Oct 3;14(6):065010. doi: 10.1088/1748-605X/ab4243. Biomed Mater. 2019. PMID: 31491773
-
miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic pre-vascularized bone formation.Biofabrication. 2021 Sep 17;13(4):10.1088/1758-5090/ac23ae. doi: 10.1088/1758-5090/ac23ae. Biofabrication. 2021. PMID: 34479220 Free PMC article.
-
3D Bioprinting for Cartilage and Osteochondral Tissue Engineering.Adv Healthc Mater. 2017 Nov;6(22). doi: 10.1002/adhm.201700298. Epub 2017 Aug 14. Adv Healthc Mater. 2017. PMID: 28804984 Review.
-
Therapeutic "Tool" in Reconstruction and Regeneration of Tissue Engineering for Osteochondral Repair.Appl Biochem Biotechnol. 2020 Jun;191(2):785-809. doi: 10.1007/s12010-019-03214-8. Epub 2019 Dec 21. Appl Biochem Biotechnol. 2020. PMID: 31863349 Review.
Cited by
-
A New Tissue Engineering Strategy to Promote Tendon-bone Healing: Regulation of Osteogenic and Chondrogenic Differentiation of Tendon-derived Stem Cells.Orthop Surg. 2024 Oct;16(10):2311-2325. doi: 10.1111/os.14152. Epub 2024 Jul 23. Orthop Surg. 2024. PMID: 39043618 Free PMC article. Review.
-
High-throughput bioprinting of spheroids for scalable tissue fabrication.Nat Commun. 2024 Nov 21;15(1):10083. doi: 10.1038/s41467-024-54504-7. Nat Commun. 2024. PMID: 39572584 Free PMC article.
-
3D bioprinted scaffolds for osteochondral regeneration: advancements and applications.Mater Today Bio. 2025 May 8;32:101834. doi: 10.1016/j.mtbio.2025.101834. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487176 Free PMC article. Review.
-
Magnetofection of miR-21 promoted by electromagnetic field and iron oxide nanoparticles via the p38 MAPK pathway contributes to osteogenesis and angiogenesis for intervertebral fusion.J Nanobiotechnology. 2023 Jan 25;21(1):27. doi: 10.1186/s12951-023-01789-3. J Nanobiotechnology. 2023. PMID: 36694219 Free PMC article.
-
Microfluidic one-step synthesis of a metal-organic framework for osteoarthritis therapeutic microRNAs delivery.Front Bioeng Biotechnol. 2023 Jul 27;11:1239364. doi: 10.3389/fbioe.2023.1239364. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37576986 Free PMC article.
References
-
- Mano JF, and Reis RL. “Osteochondral defects: present situation and tissue engineering approaches.” Journal of tissue engineering and regenerative medicine 1.4 (2007): 261–273. - PubMed
-
- Panseri Silvia, et al. “Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration.” Knee Surgery, Sports Traumatology, Arthroscopy 20.6 (2012): 1182–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
