Faecal Myeloperoxidase as a Biomarker of Endoscopic Activity in Inflammatory Bowel Disease
- PMID: 35803583
- PMCID: PMC9721461
- DOI: 10.1093/ecco-jcc/jjac098
Faecal Myeloperoxidase as a Biomarker of Endoscopic Activity in Inflammatory Bowel Disease
Abstract
Background and aims: Inflammatory bowel disease [IBD], consisting of Crohn's disease [CD] and ulcerative colitis [UC], is a relapsing-remitting illness. Treat-to-target IBD management strategies require monitoring of gastrointestinal inflammation. This study aimed to investigate faecal myeloperoxidase [fMPO], a neutrophil granule enzyme, as a biomarker of IBD activity.
Methods: Prospectively recruited participants with IBD, undergoing ileocolonoscopy for disease assessment, provided biological samples and completed symptom questionnaires prior to endoscopy. fMPO, C-reactive protein [CRP], and faecal calprotectin [fCal] were compared with validated endoscopic indices [simple endoscopic score for CD and UC endoscopic index of severity]. Receiver operating characteristic [ROC] curves assessed the performance of fMPO, CRP, and fCal in predicting endoscopic disease activity. Baseline biomarkers were used to predict a composite endpoint of complicated disease at 12 months [need for escalation of biologic/immunomodulator due to relapse, steroid use, IBD-related hospitalisation, and surgery].
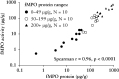
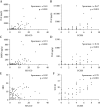
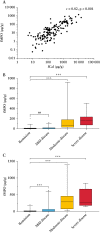
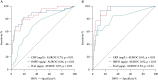
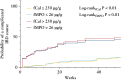
Results: A total of 172 participants were recruited [91 female, 100 with CD]. fMPO was significantly correlated with endoscopic activity in both CD [r = 0.53, p < 0.01] and UC [r = 0.63, p < 0.01], and with fCal in all patients with IBD [r = 0.82, p < 0.01]. fMPO was effective in predicting moderate-to-severely active CD [AUROC 0.86, p < 0.01] and UC [AUROC 0.92, p < 0.01]. Individuals with a baseline fMPO > 26 µg/g were significantly more likely to reach the composite outcome at 12 months (hazard ratio [HR] 3.71, 95% confidence interval [CI] 2.07-6.64, p < 0.01).
Conclusions: Faecal myeloperoxidase is an accurate biomarker of endoscopic activity in IBD and predicted a more complicated IBD course during follow-up.
Keywords: Biomarkers; myeloperoxidase; prognosis.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures

Comment in
-
Evaluating Discriminative Accuracy of Biomarkers in Relation to Binary Study Outcomes: First Validate, Then Celebrate?J Crohns Colitis. 2023 Jan 27;17(1):146. doi: 10.1093/ecco-jcc/jjac117. J Crohns Colitis. 2023. PMID: 35962957 No abstract available.
References
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] Initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. - PubMed
-
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease [CALM]: a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779–89. - PubMed
-
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. - PubMed
-
- Annese V, Daperno M, Rutter MD, et al. European evidence-based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982–1018. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

