Does awake prone positioning prevent the use of mechanical respiratory support or death in COVID-19 patients on standard oxygen therapy hospitalised in general wards? A multicentre randomised controlled trial: the PROVID-19 protocol
- PMID: 35803621
- PMCID: PMC9271841
- DOI: 10.1136/bmjopen-2021-060320
Does awake prone positioning prevent the use of mechanical respiratory support or death in COVID-19 patients on standard oxygen therapy hospitalised in general wards? A multicentre randomised controlled trial: the PROVID-19 protocol
Abstract
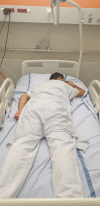
Introduction: COVID-19 is responsible of severe hypoxaemia and acute respiratory distress syndrome (ARDS). Prone positioning improves oxygenation and survival in sedated mechanically patients with ARDS not related to COVID-19. Awake prone positioning is a simple and safe technique which improves oxygenation in non-intubated COVID-19 patients. We hypothesised that early prone positioning in COVID-19 patients breathing spontaneously in medical wards could decrease the rates of intubation or need for noninvasive ventilation or death.
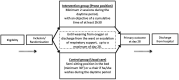
Methods and analysis: PROVID-19 is an investigator-initiated, prospective, multicentre randomised, controlled, superiority trial comparing awake prone positioning to standard of care in hypoxaemic COVID-19 patients in 20 medical wards in France and Monaco. Patients are randomised to receive either awake prone position plus usual care or usual care alone with stratification on centres, body mass index and severity of hypoxaemia.The study objective is to compare the rate of treatment failure defined as a composite endpoint comprising the need for non-invasive ventilation (at two pressure levels) or for intubation or death, between the intervention group (awake prone position plus usual care) and the usual care (usual care alone) group at 28 days.
Ethics and dissemination: The protocol and amendments have been approved by the ethics committees (Comité de protection des personnes Ouest VI, France, no 1279 HPS2 and Comité Consultatif d'Ethique en matière de Recherche Biomédicale, Monaco, no 2020.8894 AP/jv), and patients are included after written informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number: NCT04363463.
Keywords: COVID-19; infectious diseases; respiratory infections.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: M-AN declares personal fees and non-financial support from Fisher & Paykel Healthcare.
Figures
References
-
- Jayakumar D, Ramachandran Dnb P, Rabindrarajan Dnb E, et al. . Standard care versus awake prone position in adult Nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 Infection-A multicenter feasibility randomized controlled trial. J Intensive Care Med 2021;36:918–24. 10.1177/08850666211014480 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials