Effectiveness and protection duration of Covid-19 vaccines and previous infection against any SARS-CoV-2 infection in young adults
- PMID: 35803915
- PMCID: PMC9263799
- DOI: 10.1038/s41467-022-31469-z
Effectiveness and protection duration of Covid-19 vaccines and previous infection against any SARS-CoV-2 infection in young adults
Abstract
Data on effectiveness and protection duration of Covid-19 vaccines and previous infection against general SARS-CoV-2 infection in general populations are limited. Here we evaluate protection from Covid-19 vaccination (primary series) and previous infection in 21,261 university students undergoing repeated surveillance testing between 8/8/2021-12/04/2021, during which B.1.617 (delta) was the dominant SARS-CoV-2 variant. Estimated mRNA-1273, BNT162b2, and AD26.COV2.S effectiveness against any SARS-CoV-2 infection is 75.4% (95% CI: 70.5-79.5), 65.7% (95% CI: 61.1-69.8), and 42.8% (95% CI: 26.1-55.8), respectively. Among previously infected individuals, protection is 72.9% when unvaccinated (95% CI: 66.1-78.4) and increased by 22.1% with full vaccination (95% CI: 15.8-28.7). Statistically significant decline in protection is observed for mRNA-1273 (P < .001), BNT162b2 (P < .001), but not Ad26.CoV2.S (P = 0.40) or previous infection (P = 0.12). mRNA vaccine protection dropped 29.7% (95% CI: 17.9-41.6) six months post- vaccination, from 83.2% to 53.5%. We conclude that the 2-dose mRNA vaccine series initially offers strong protection against general SARS-CoV-2 infection caused by the delta variant in young adults, but protection substantially decreases over time. These findings indicate that vaccinated individuals may still contribute to community spread. While previous SARS-CoV-2 infection consistently provides moderately strong protection against repeat infection from delta, vaccination yields a substantial increase in protection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
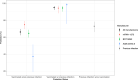
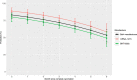
References
-
- Thompson, M. G. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers —eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 70. 10.15585/mmwr.mm7013e3 (2021). - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

