Polymicrobial biofilms of ocular bacteria and fungi on ex vivo human corneas
- PMID: 35803992
- PMCID: PMC9270462
- DOI: 10.1038/s41598-022-15809-z
Polymicrobial biofilms of ocular bacteria and fungi on ex vivo human corneas
Abstract
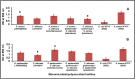
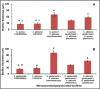
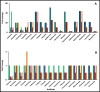
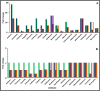
Microbes residing in biofilms confer several fold higher antimicrobial resistances than their planktonic counterparts. Compared to monomicrobial biofilms, polymicrobial biofilms involving multiple bacteria, multiple fungi or both are more dominant in nature. Paradoxically, polymicrobial biofilms are less studied. In this study, ocular isolates of Staphylococcus aureus, S. epidermidis and Candida albicans, the etiological agents of several ocular infections, were used to demonstrate their potential to form mono- and polymicrobial biofilms both in vitro and on human cadaveric corneas. Quantitative (crystal violet and XTT methods) and qualitative (confocal and scanning electron microscopy) methods demonstrated that they form polymicrobial biofilms. The extent of biofilm formation was dependent on whether bacteria and fungi were incubated simultaneously or added to a preformed biofilm. Additionally, the polymicrobial biofilms exhibited increased resistance to different antimicrobials compared to planktonic cells. When the MBECs of different antibacterial and antifungal agents were monitored it was observed that the MBECs in the polymicrobial biofilms was either identical or decreased compared to the monomicrobial biofilms. The results are relevant in planning treatment strategies for the eye. This study demonstrates that ocular bacteria and fungi form polymicrobial biofilms and exhibit increase in antimicrobial resistance compared to the planktonic cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

