Intracellular development and impact of a marine eukaryotic parasite on its zombified microalgal host
- PMID: 35804051
- PMCID: PMC9478091
- DOI: 10.1038/s41396-022-01274-z
Intracellular development and impact of a marine eukaryotic parasite on its zombified microalgal host
Abstract
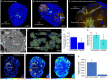
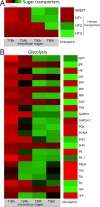
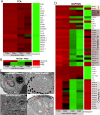
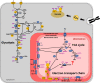
Parasites are widespread and diverse in oceanic plankton and many of them infect single-celled algae for survival. How these parasites develop and scavenge energy within the host and how the cellular organization and metabolism of the host is altered remain open questions. Combining quantitative structural and chemical imaging with time-resolved transcriptomics, we unveil dramatic morphological and metabolic changes of the marine parasite Amoebophrya (Syndiniales) during intracellular infection, particularly following engulfment and digestion of nutrient-rich host chromosomes. Changes include a sequential acristate and cristate mitochondrion with a 200-fold increase in volume, a 13-fold increase in nucleus volume, development of Golgi apparatus and a metabolic switch from glycolysis (within the host) to TCA (free-living dinospore). Similar changes are seen in apicomplexan parasites, thus underlining convergent traits driven by metabolic constraints and the infection cycle. In the algal host, energy-producing organelles (plastid, mitochondria) remain relatively intact during most of the infection. We also observed that sugar reserves diminish while lipid droplets increase. Rapid infection of the host nucleus could be a "zombifying" strategy, allowing the parasite to digest nutrient-rich chromosomes and escape cytoplasmic defense, whilst benefiting from maintained carbon-energy production of the host cell.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Worden AZ, Follows MJ, Giovannoni SJ, Wilken S, Zimmerman AE, Keeling PJ. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science. 2015;347:1257594–1257594. - PubMed
-
- Guillou L, Viprey M, Chambouvet A, Welsh RM, Kirkham AR, Massana R, et al. Widespread occurrence and genetic diversity of marine parasitoids belonging to Syndiniales (Alveolata) Environ Microbiol. 2008;10:3349–65. - PubMed
-
- Jephcott TG, Alves-de-Souza C, Gleason FH, van Ogtrop FF, Sime-Ngando T, Karpov SA, et al. Ecological impacts of parasitic chytrids, syndiniales and perkinsids on populations of marine photosynthetic dinoflagellates. Fungal Ecol. 2016;19:47–58.
-
- Alacid E, Reñé A, Garcés E. New Insights into the Parasitoid Parvilucifera sinerae Life Cycle: The Development and Kinetics of Infection of a Bloom-forming Dinoflagellate Host. Protist. 2015;166:677–99. - PubMed
-
- Not F, Gausling R, Azam F, Heidelberg JF, Worden AZ. Vertical distribution of picoeukaryotic diversity in the Sargasso Sea. Environ Microbiol. 2007;9:1233–52. - PubMed

