Identification of the major photodegradant in metronidazole by LC-PDA-MS and its reveal in compendial methods
- PMID: 35804169
- PMCID: PMC9270378
- DOI: 10.1038/s41598-022-15625-5
Identification of the major photodegradant in metronidazole by LC-PDA-MS and its reveal in compendial methods
Abstract
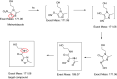
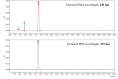
Metronidazole in aqueous solution is sensitive to light and UV irradiation, leading to the formation of N-(2-hydroxyethyl)-5-methyl-l,2,4-oxadiazole-3-carboxamide. This is revealed here by liquid chromatography with tandem photo diode array detection and mass spectrometry (LC-PDA-MS) and further verified by comparison with the corresponding reference substance and proton nuclear magnetic resonance (1H-NMR). However, in current compendial tests for related substances/organic impurities of metronidazole, the above photolytic degradant could not be detected. Thus, when photodegradation of metronidazole occurs, it could not be demonstrated. In our study, an improved LC method was developed and validated, which includes a detection at a wavelength of 230 nm and optimization of mobile phase composition thereby a better separation was obtained.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Study of the structures of photodegradation impurities and pathways of photodegradation of cilnidipine by liquid chromatography/Q-Orbitrap mass spectrometry.Rapid Commun Mass Spectrom. 2016 Aug 15;30(15):1771-8. doi: 10.1002/rcm.7657. Rapid Commun Mass Spectrom. 2016. PMID: 27426453
-
LC and LC-MS/MS study of forced decomposition behavior of anastrozole and establishment of validated stability-indicating analytical method for impurities estimation in low dose anastrozole tablets.J Pharm Biomed Anal. 2009 Oct 15;50(3):397-404. doi: 10.1016/j.jpba.2009.05.025. Epub 2009 May 30. J Pharm Biomed Anal. 2009. PMID: 19541446
-
Automated Solid Phase Extraction (SPE) LC/NMR Applied to the Structural Analysis of Extractable Compounds from a Pharmaceutical Packaging Material of Construction.PDA J Pharm Sci Technol. 2013 May-Jun;67(3):267-87. doi: 10.5731/pdajpst.2013.00920. PDA J Pharm Sci Technol. 2013. PMID: 23752753
-
Critical practical aspects in the application of liquid chromatography-mass spectrometric studies for the characterization of impurities and degradation products.J Pharm Biomed Anal. 2014 Jan;87:191-217. doi: 10.1016/j.jpba.2013.04.027. Epub 2013 Apr 28. J Pharm Biomed Anal. 2014. PMID: 23706957 Review.
References
-
- Wilkins BJ, Gainsford GJ, Moore DE. Photolytic rearrangement of metronidazole to N-(2-hydroxyethyl)-5-methyl-l,2,4-oxadiazole-3-carboxamide. Crystal structure of its 4-nitrobenzoate derivative. J. Chem. Soc. Perkin Trans. 1987;I:1817–1820. doi: 10.1039/p19870001817. - DOI
-
- Pfoertner KH, Daly JJ. Photochemical rearrangement of N-substituted 2-methy-5- nitro-1H-imidazoles in the presence of water. Helv. Chim. Acta. 1987;70:171–174. doi: 10.1002/hlca.19870700121. - DOI
-
- Wilkins BJ, Moore DE. Photolytic rearrangement of metronidazole to 1-hydroxyethyl-2-methyl-4-hydroxyimino-5-oxo-imidazole and the formation of copper complexes of these compounds. Photochem. Photobiol. 1988;47(4):481–484. doi: 10.1111/j.1751-1097.1988.tb08834.x. - DOI
-
- Moore DE, Wilkins BJ. Common products from gamma-radiolysis and ultraviolet photolysis of metronidazole. Radiat. Phys. Chem. 1990;36(4):547–550.
-
- Moore DE, Chignell CF, Sik RH, Motten AG. Generation of radical anions from metronidazole, misonidazole and azathioprine by photoreduction in the presence of EDTA. Int. J. Radiat. Biol. 1986;50(5):885–891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

