Mechanisms of mitochondrial respiratory adaptation
- PMID: 35804199
- PMCID: PMC9926497
- DOI: 10.1038/s41580-022-00506-6
Mechanisms of mitochondrial respiratory adaptation
Abstract
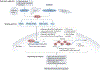
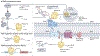
Mitochondrial energetic adaptations encompass a plethora of conserved processes that maintain cell and organismal fitness and survival in the changing environment by adjusting the respiratory capacity of mitochondria. These mitochondrial responses are governed by general principles of regulatory biology exemplified by changes in gene expression, protein translation, protein complex formation, transmembrane transport, enzymatic activities and metabolite levels. These changes can promote mitochondrial biogenesis and membrane dynamics that in turn support mitochondrial respiration. The main regulatory components of mitochondrial energetic adaptation include: the transcription coactivator peroxisome proliferator-activated receptor-γ (PPARγ) coactivator 1α (PGC1α) and associated transcription factors; mTOR and endoplasmic reticulum stress signalling; TOM70-dependent mitochondrial protein import; the cristae remodelling factors, including mitochondrial contact site and cristae organizing system (MICOS) and OPA1; lipid remodelling; and the assembly and metabolite-dependent regulation of respiratory complexes. These adaptive molecular and structural mechanisms increase respiration to maintain basic processes specific to cell types and tissues. Failure to execute these regulatory responses causes cell damage and inflammation or senescence, compromising cell survival and the ability to adapt to energetically demanding conditions. Thus, mitochondrial adaptive cellular processes are important for physiological responses, including to nutrient availability, temperature and physical activity, and their failure leads to diseases associated with mitochondrial dysfunction such as metabolic and age-associated diseases and cancer.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Gilkerson RW, Selker JML & Capaldi RA The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 546, 355–358 (2003). - PubMed
-
- Deshpande OA & Mohiuddin SS Biochemistry, Oxidative Phophorylation. StatPearls (2020). - PubMed
-
- Walker JE The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans 41, 1–16 (2013). - PubMed
-
- Enerbäck S et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

