DHCR24 Knockdown Induces Tau Hyperphosphorylation at Thr181, Ser199, Ser262, and Ser396 Sites via Activation of the Lipid Raft-Dependent Ras/MEK/ERK Signaling Pathway in C8D1A Astrocytes
- PMID: 35804281
- PMCID: PMC9395500
- DOI: 10.1007/s12035-022-02945-w
DHCR24 Knockdown Induces Tau Hyperphosphorylation at Thr181, Ser199, Ser262, and Ser396 Sites via Activation of the Lipid Raft-Dependent Ras/MEK/ERK Signaling Pathway in C8D1A Astrocytes
Abstract
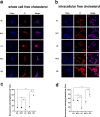
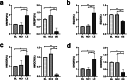
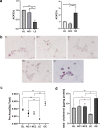
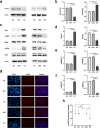
The synthetase 3β-hydroxysterol-Δ24 reductase (DHCR24) is a key regulator involved in cholesterol synthesis and homeostasis. A growing body of evidence indicates that DHCR24 is downregulated in the brain of various models of Alzheimer's disease (AD), such as astrocytes isolated from AD mice. For the past decades, astrocytic tau pathology has been found in AD patients, while the origin of phosphorylated tau in astrocytes remains unknown. A previous study suggests that downregulation of DHCR24 is associated with neuronal tau hyperphosphorylation. Herein, the present study is to explore whether DHCR24 deficiency can also affect tau phosphorylation in astrocytes. Here, we showed that DHCR24 knockdown could induce tau hyperphosphorylation at Thr181, Ser199, Thr231, Ser262, and Ser396 sites in C8D1A astrocytes. Meanwhile, we found that DHCR24-silencing cells had reduced the level of free cholesterol in the plasma membrane and intracellular organelles, as well as cholesterol esters. Furthermore, reduced cellular cholesterol level caused a decreased level of the caveolae-associated protein, cavin1, which disrupted lipid rafts/caveolae and activated rafts/caveolae-dependent Ras/MEK/ERK signaling pathway. In contrast, overexpression of DHCR24 prevented the overactivation of Ras/MEK/ERK signaling by increasing cellular cholesterol content, therefore decreasing tau hyperphosphorylation in C8D1A astrocytes. Herein, we firstly found that DHCR24 knockdown can lead to tau hyperphosphorylation in the astrocyte itself by activating lipid raft-dependent Ras/MEK/ERK signaling, which might contribute to the pathogenesis of AD and other degenerative tauopathies.
Keywords: Alzheimer’s disease; Astrocyte; Cholesterol; DHCR24; Tau hyperphosphorylation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
DHCR24 Knockdown Lead to Hyperphosphorylation of Tau at Thr181, Thr231, Ser262, Ser396, and Ser422 Sites by Membrane Lipid-Raft Dependent PP2A Signaling in SH-SY5Y Cells.Neurochem Res. 2021 Jul;46(7):1627-1640. doi: 10.1007/s11064-021-03273-6. Epub 2021 Mar 12. Neurochem Res. 2021. PMID: 33710538
-
DHCR24 Knock-Down Induced Tau Hyperphosphorylation at Thr181, Ser199, Thr231, Ser262, Ser396 Epitopes and Inhibition of Autophagy by Overactivation of GSK3β/mTOR Signaling.Front Aging Neurosci. 2021 Apr 21;13:513605. doi: 10.3389/fnagi.2021.513605. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33967735 Free PMC article.
-
The role of DHCR24 in the pathogenesis of AD: re-cognition of the relationship between cholesterol and AD pathogenesis.Acta Neuropathol Commun. 2022 Mar 16;10(1):35. doi: 10.1186/s40478-022-01338-3. Acta Neuropathol Commun. 2022. PMID: 35296367 Free PMC article. Review.
-
Cellular Cholesterol Loss Impairs Synaptic Vesicle Mobility via the CAMK2/Synapsin-1 Signaling Pathway.Front Biosci (Landmark Ed). 2025 Jan 20;30(1):27111. doi: 10.31083/FBL27111. Front Biosci (Landmark Ed). 2025. PMID: 39862102
-
Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis.Prog Lipid Res. 2013 Oct;52(4):666-80. doi: 10.1016/j.plipres.2013.09.002. Epub 2013 Oct 2. Prog Lipid Res. 2013. PMID: 24095826 Review.
Cited by
-
DHCR24 reverses Alzheimer's disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice.Acta Neuropathol Commun. 2023 Jun 21;11(1):102. doi: 10.1186/s40478-023-01593-y. Acta Neuropathol Commun. 2023. PMID: 37344916 Free PMC article.
-
Mesenchymal stem cell application in Alzheimer's disease.Regen Ther. 2025 Jul 26;30:439-445. doi: 10.1016/j.reth.2025.07.006. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40756017 Free PMC article. Review.
-
Lipidome disruption in Alzheimer's disease brain: detection, pathological mechanisms, and therapeutic implications.Mol Neurodegener. 2025 Jan 27;20(1):11. doi: 10.1186/s13024-025-00803-6. Mol Neurodegener. 2025. PMID: 39871348 Free PMC article. Review.
-
Navigating the metabolic maze: anomalies in fatty acid and cholesterol processes in Alzheimer's astrocytes.Alzheimers Res Ther. 2024 Mar 23;16(1):63. doi: 10.1186/s13195-024-01430-x. Alzheimers Res Ther. 2024. PMID: 38521950 Free PMC article. Review.
-
American Ginseng for the Treatment of Alzheimer's Disease: A Review.Molecules. 2023 Jul 28;28(15):5716. doi: 10.3390/molecules28155716. Molecules. 2023. PMID: 37570686 Free PMC article. Review.
References
-
- Chiarini A, Armato U, Gardenal E, Gui L, Dal Prà I. Amyloid β-exposed human astrocytes overproduce phospho-tau and overrelease it within exosomes, effects suppressed by calcilytic NPS 2143-further implications for Alzheimer’s therapy. Front Neurosci. 2017;11:217. doi: 10.3389/fnins.2017.00217. - DOI - PMC - PubMed
-
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/jneurosci.1860-14.2014. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

