Perioperative levetiracetam for seizure prophylaxis in seizure-naive brain tumor patients with focus on neurocognitive functioning
- PMID: 35804291
- PMCID: PMC9264633
- DOI: 10.1186/s12883-022-02762-7
Perioperative levetiracetam for seizure prophylaxis in seizure-naive brain tumor patients with focus on neurocognitive functioning
Abstract
Introduction: In seizure-naive brain tumor patients, the efficacy of perioperative prophylactic antiepileptic drug treatment remains controversial. In case of administration, the common preferred drug is levetiracetam (LEV) because of its favorable pharmacological profile. Research to date has not sufficiently determined how LEV affects cognition in the short term, as is the case in the perioperative period. The objective of this prospective study was to examine the neurocognitive functioning of seizure-naive brain tumor patients after receiving LEV perioperatively.
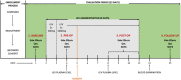
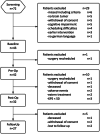
Methods: Fortythree patients with supratentorial brain tumor scheduled for surgery received LEV three days before until six days after surgery as seizure prophylaxis. Cognitive functioning (NeuroCogFX), LEV plasma-levels, hematotoxicity, side-effects, as well as health-related quality of life (HRQoL, Qolie31), were recorded preoperatively before (Baseline) and after onset of LEV (Pre-Op), 4-6 days postoperatively (Post-Op) and 21 days postoperatively (Follow-Up).
Results: No significant changes in cognitive functioning and HRQoL were seen after onset of preoperative LEV. There was a significant improvement of NeuroCogFX total-score at Follow-Up (p = 0.004) compared to Baseline. The overall-score Qolie31 showed simultaneous improvement patterns as cognitive functioning (p < 0.001). The most frequent side effect related to study drug was somnolence (in 28.6% of patients).
Conclusions: A significant improvement of cognitive functioning, as well as an improvement in HRQoL, were detected postoperatively. This is presumably due to the debulking effect of the surgery. Nevertheless, LEV has no detrimental effect on cognitive functioning in the perioperative phase in seizure-naive brain tumor patients.
Trial registration: This study was registered prospectively (Date: 25/11/2015; EudraCT: 2015-003,916-19).
Keywords: Brain tumor; Cognition; Cognitive functioning; Perioperative seizure prophylaxis; Surgery.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Glantz, M.J., et al., Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 2000. 54(10): p. 1886–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

