The long-term effect of mTOR inhibition on lipid and glucose metabolism in tuberous sclerosis complex: data from the Dutch TSC registry
- PMID: 35804402
- PMCID: PMC9264703
- DOI: 10.1186/s13023-022-02385-8
The long-term effect of mTOR inhibition on lipid and glucose metabolism in tuberous sclerosis complex: data from the Dutch TSC registry
Abstract
Background: MTOR inhibition is an effective treatment for many manifestations of tuberous sclerosis complex. Because mTOR inhibition is a disease modifying therapy, lifelong use will most likely be necessary. This study addresses the long-term effects of mTOR inhibitors on lipid and glucose metabolism and aims to provide better insight in the incidence and time course of these metabolic adverse effects in treated TSC patients.
Methods: All patients who gave informed consent for the nationwide TSC Registry and were ever treated with mTOR inhibitors (sirolimus and/or everolimus) were included. Lipid profiles, HbA1c and medication were analysed in all patients before and during mTOR inhibitor treatment.
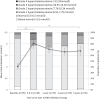
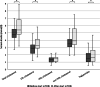
Results: We included 141 patients, the median age was 36 years, median use of mTOR inhibitors 5.1 years (aimed serum levels 3.0-5.0 µg/l). Total cholesterol, LDL- and HDL-cholesterol levels at baseline were similar to healthy reference data. After start of mTOR inhibition therapy, total cholesterol, LDL-cholesterol and triglycerides increased significantly and were higher compared to healthy reference population. Mean total cholesterol levels increased by 1.0 mmol/L after 3-6 months of mTOR inhibition therapy but did not increase further during follow-up. In this study, 2.5% (3/118) of patients developed diabetes (defined as an HbA1c ≥ 48 mmol/mol) during a median follow-up of 5 years.
Conclusions: Hypercholesterolemia is a frequent side effect of mTOR inhibition in TSC patients, and predominantly occurs within the first year of treatment. Although hyperglycemia is a frequent side effect in other indications for mTOR inhibition, incidence of diabetes mellitus in TSC patients was only 2.5%. This may reflect the difference of mTOR inhibition in patients with normal mTOR complex pathway function versus patients with overactive mTOR complex signaling due to a genetic defect (TSC patients).
Keywords: Adverse effects; Diabetes mellitus; Dyslipidemia; Hypercholesterolemia; Hyperglycemia; Long-term; Tuberous sclerosis complex; mTOR inhibition.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

