Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial
- PMID: 35804491
- PMCID: PMC9544523
- DOI: 10.1111/clr.13972
Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial
Erratum in
-
Corrigendum.Clin Oral Implants Res. 2023 Jan;34(1):78-79. doi: 10.1111/clr.14021. Epub 2022 Dec 11. Clin Oral Implants Res. 2023. PMID: 36504458 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the potential benefit of the use of a bone substitute material in the reconstructive surgical therapy of peri-implantitis.
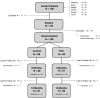
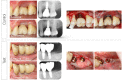
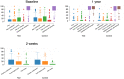
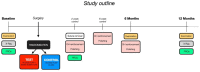
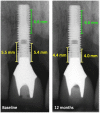
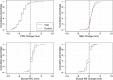
Methods: In this multicenter randomized clinical trial, 138 patients (147 implants) with peri-implantitis were treated surgically, randomized by coin toss to either a control (access flap surgery) or a test group (reconstructive surgery using bone substitute material). Clinical assessments, including probing pocket depth (PPD), bleeding and suppuration on probing (BOP & SOP) as well as soft tissue recession (REC), were recorded at baseline, 6 and 12 months. Marginal bone levels (MBL), measured on intra-oral radiographs, and patient-reported outcomes (PROs) were recorded at baseline and 12 months. No blinding to group allocation was performed. The primary outcome at 12 months was a composite measure including (i) implant not lost, (ii) absence of BOP/SOP at all aspects, (iii) PPD ≤5 mm at all aspects and (iv) ≤1 mm recession of mucosal margin on the buccal aspect of the implant. Secondary outcomes included (i) changes of MBL, (ii) changes of PPD, BOP%, and buccal KM, (iii) buccal REC and (iv) patient-reported outcomes.
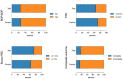
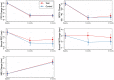
Results: During follow-up, four implants (one in the test group, three in the control group) in four patients were removed due to disease progression. At 12 months, a total of 69 implants in the test and 68 implants in the control group were examined. Thus, 16.4% and 13.5% of implants in the test and control group, respectively, met all predefined criteria of the composite outcome. PPD reduction and MBL gain were 3.7 mm and about 1.0 mm in both groups. Reduction in mean BOP% varied between 45% (test) and 50% (control), without significant differences between groups. Buccal REC was less pronounced in the test group (M = 0.7, SD = 0.9 mm) when compared to controls (M = 1.1, SD = 1.5 mm). PROs were favorable in both groups without significant differences. One case of allergic reaction to the antibiotic therapy was recorded. No other adverse events were noted.
Conclusions: Surgical therapy of peri-implantitis effectively improved the clinical and radiographic status at 12 months. While the use of a bone substitute material did not improve reductions of PPD and BOP, buccal REC was less pronounced in the test group. Patient satisfaction was high in both groups.
Keywords: bone graft; dental implant; peri-implantitis; reconstructive therapy; surgical therapy.
© 2022 The Authors. Clinical Oral Implants Research published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Derks reports speakers honoraria from Osteology Foundation, Dentsply Sirona Implants, Straumann Group and received research grants from Eklund Foundation and Electro Medical Systems. Dr. Ortiz‐Vigón reports speakers honoraria from Straumann Group and Arrow Development research and financial support from Thinking Perio research. Dr. Guerrero reports honoraria from Inibsa and Dentsply Sirona Implants. Dr. Donati reports speakers honoraria from Dentsply Sirona Implants and received research grants from Dentsply Sirona Implants. Dr. Bressan reports speakers honoraria from Dentsply Sirona Implants and Sweden & Martina. Dr. Ghensi reports speakers honoraria from Geistlich Pharma AG and BioHorizons Camlog. Dr. Schaller reports speakers honoraria from Zimmer Biomet. Dr. Tomasi reports speakers honoraria from Dentsply Sirona Implants, Straumann Group, Geistlich Pharma AG and Sweden & Martina. Dr. Karlsson reports speakers honoraria from Dentsply Sirona Implants. Dr. Abrahamsson received research grants from Dentsply Sirona Implants. Dr. Berglundh reports honoraria from Dentsply Sirona Implants, speakers honoraria from Osteology Foundation and received research grants from Dentsply Sirona Implants and Geistlich Pharma AG.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

