Tissue Distribution of the Piscine Novirhabdovirus Genotype IVb in Muskellunge (Esox masquinongy)
- PMID: 35804529
- PMCID: PMC9264975
- DOI: 10.3390/ani12131624
Tissue Distribution of the Piscine Novirhabdovirus Genotype IVb in Muskellunge (Esox masquinongy)
Abstract
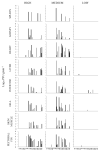
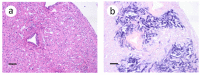
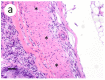
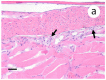
A novel sublineage of the piscine novirhabdovirus (synonym: viral hemorrhagic septicemia virus), genotype IVb, emerged in the Laurentian Great Lakes, causing serious losses in resident fish species as early as 2003. Experimentally infected juvenile muskellunge (Esox masquinongy) were challenged with VHSV-IVb at high (1 × 105 PFU mL-1), medium (4 × 103 PFU mL-1), and low (100 PFU mL-1) doses. Samples from spleen, kidneys, heart, liver, gills, pectoral fin, large intestine, and skin/muscle were collected simultaneously from four fish at each predetermined time point and processed for VHSV-IVb reisolaton on Epitheliosum papulosum cyprini cell lines and quantification by plaque assay. The earliest reisolation of VHSV-IVb occurred in one fish from pectoral fin samples at 24 h post-infection. By 6 days post-infection (dpi), all tissue types were positive for VHSV-IVb. Statistical analysis suggested that virus levels were highest in liver, heart, and skin/muscle samples. In contrast, the kidneys and spleen exhibited reduced probability of virus recovery. Virus distribution was further confirmed by an in situ hybridization assay using a VHSV-IVb specific riboprobe. Heart muscle fibers, hepatocytes, endothelia, smooth muscle cells, and fibroblast-like cells of the pectoral fin demonstrated riboprobe labeling, thus highlighting the broad cellular tropism of VHSV-IVb. Histopathologic lesions were observed in areas where the virus was visualized.
Keywords: in situ hybridization; muskellunge; pathogenesis; tropism; viral hemorrhagic septicemia virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Shedding of viral hemorrhagic septicemia virus (Genotype IVb) by experimentally infected muskellunge (Esox masquinongy).J Microbiol. 2012 Apr;50(2):278-84. doi: 10.1007/s12275-012-1145-2. Epub 2012 Apr 27. J Microbiol. 2012. PMID: 22538657
-
Production of a monoclonal antibody against of muskellunge (Esox masquinongy) IgM heavy chain and its use in development of an indirect ELISA for titrating circulating antibodies against VHSV-IVB.Fish Shellfish Immunol. 2019 May;88:464-471. doi: 10.1016/j.fsi.2019.03.002. Epub 2019 Mar 8. Fish Shellfish Immunol. 2019. PMID: 30858097
-
Detection of viral hemorrhagic septicemia virus-IVb antibodies in sera of muskellunge Esox masquinongy using competitive ELISA.Dis Aquat Organ. 2014 Apr 3;108(3):187-99. doi: 10.3354/dao02712. Dis Aquat Organ. 2014. PMID: 24695232
-
DNA Vaccination Partially Protects Muskellunge against Viral Hemorrhagic Septicemia Virus (VHSV-IVb).J Aquat Anim Health. 2017 Mar;29(1):50-56. doi: 10.1080/08997659.2016.1238413. J Aquat Anim Health. 2017. PMID: 28225652
-
Comparative susceptibility of representative Great Lakes fish species to the North American viral hemorrhagic septicemia virus Sublineage IVb.Dis Aquat Organ. 2010 Jul 26;91(1):23-34. doi: 10.3354/dao02217. Dis Aquat Organ. 2010. PMID: 20853739
Cited by
-
Effects of Escherichia coli strain Nissle 1917 on arsenic-challenged goldfish (Carassius auratus): histological evidence.Vet Res Forum. 2023;14(7):367-372. doi: 10.30466/vrf.2022.557449.3551. Epub 2023 Jul 15. Vet Res Forum. 2023. PMID: 37564358 Free PMC article.
References
-
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Dempsey D.M., Dutilh B.E., Harrach B., Harrison R.L., Hendrickson R.C., et al. Changes to virus taxonomy and the statutes ratified by the international committee on taxonomy of viruses. Arch. Virol. 2020;165:2737–2748. doi: 10.1007/s00705-020-04752-x. - DOI - PubMed
-
- Wolf K. Viral Hemorrhagic Septicemia. In: Wolf K., editor. Fish Viruses and Fish Viral Diseases. Comstock Publishing Associates; Cornell University Press, Ithaca, NY, USA and London, UK: 1988. pp. 217–249.
-
- Smail D.A. Viral haemorrhagic septicaemia. In: Woo P., Bruno D.W., editors. Fish Diseases and Disorders: Viral, Bacterial, and Fungal Infections. Vol. 3. CABI Publishing; Wallingford, Oxfordshire, UK: 1999. pp. 123–146.
-
- Elsayed E., Faisal M., Thomas G., Whelan G., Batts W., Winton J. Isolation of viral haemorrhagic septicaemia virus from muskellunge, Esox masquinongy (Mitchill), in Lake St. Clair, Michigan, USA reveals a new sublineage of the North American genotype. J. Fish Dis. 2006;29:611–619. doi: 10.1111/j.1365-2761.2006.00755.x. - DOI - PubMed
-
- Faisal M., Ahne W. Spring viremia of carp virus (SVCV): Comparison of immunoperoxidase, fluorescent antibody and cell culture isolation techniques for detection of antigen. J. Fish Dis. 1984;7:57–64. doi: 10.1111/j.1365-2761.1984.tb00906.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

