Comparative Proteomic Analyses of Poorly Motile Swamp Buffalo Spermatozoa Reveal Low Energy Metabolism and Deficiencies in Motility-Related Proteins
- PMID: 35804605
- PMCID: PMC9264820
- DOI: 10.3390/ani12131706
Comparative Proteomic Analyses of Poorly Motile Swamp Buffalo Spermatozoa Reveal Low Energy Metabolism and Deficiencies in Motility-Related Proteins
Abstract
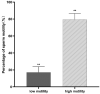
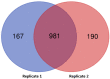
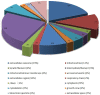
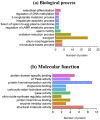
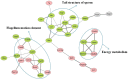
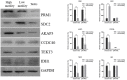
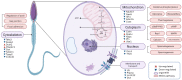
The acquisition of mammalian sperm motility is a main indicator of epididymal sperm maturation and helps ensure fertilization. Poor sperm motility will prevent sperm cells from reaching the fertilization site, resulting in fertilization failure. To investigate the proteomic profiling of normal and poorly motile buffalo spermatozoa, a strategy applying liquid chromatography tandem mass spectrometry combined with tandem mass targeting was used. As a result, 145 differentially expressed proteins (DEPs) were identified in poorly motile spermatozoa (fold change > 1.5), including 52 upregulated and 93 downregulated proteins. The upregulated DEPs were mainly involved in morphogenesis and regulation of cell differentiation. The downregulated DEPs were involved with transport, oxidation-reduction, sperm motility, regulation of cAMP metabolism and regulation of DNA methylation. The mRNA and protein levels of PRM1 and AKAP3 were lower in poorly motile spermatozoa, while the expressions of SDC2, TEKT3 and IDH1 were not correlated with motility, indicating that their protein changes were affected by transcription or translation. Such changes in the expression of these proteins suggest that the formation of poorly motile buffalo spermatozoa reflects a low efficiency of energy metabolism, decreases in sperm protamine proteins, deficiencies in motility-related proteins, and variations in tail structural proteins. Such proteins could be biomarkers of poorly motile spermatozoa. These results illustrate some of the molecular mechanisms associated with poorly motile spermatozoa and provide clues for finding molecular markers of these pathways.
Keywords: LC-MS/MS; buffalo; proteomics; sperm motility; tandem mass targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparative proteomic analysis of seminal plasma exosomes in buffalo with high and low sperm motility.BMC Genomics. 2023 Jan 9;24(1):8. doi: 10.1186/s12864-022-09106-2. BMC Genomics. 2023. PMID: 36624393 Free PMC article.
-
Comparative proteomic identification buffalo spermatozoa during in vitro capacitation.Theriogenology. 2019 Mar 1;126:303-309. doi: 10.1016/j.theriogenology.2018.12.025. Epub 2018 Dec 15. Theriogenology. 2019. PMID: 30599421
-
Expression of cytochrome P450 aromatase transcripts in buffalo (Bubalus bubalis)-ejaculated spermatozoa and its relationship with sperm motility.Domest Anim Endocrinol. 2008 Apr;34(3):238-49. doi: 10.1016/j.domaniend.2007.07.003. Epub 2007 Aug 20. Domest Anim Endocrinol. 2008. PMID: 17851018
-
Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals.Reprod Biol Endocrinol. 2018 Feb 27;16(1):16. doi: 10.1186/s12958-018-0334-1. Reprod Biol Endocrinol. 2018. PMID: 29482568 Free PMC article. Review.
-
Role(s) of the serine/threonine protein phosphatase 1 on mammalian sperm motility.Arch Androl. 2007 Jul-Aug;53(4):169-77. doi: 10.1080/01485010701314032. Arch Androl. 2007. PMID: 17852041 Review.
Cited by
-
Determinant genetic markers of semen quality in livestock.Front Endocrinol (Lausanne). 2024 Oct 4;15:1456305. doi: 10.3389/fendo.2024.1456305. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39429738 Free PMC article. Review.
-
Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis.Int J Mol Sci. 2024 Apr 8;25(7):4121. doi: 10.3390/ijms25074121. Int J Mol Sci. 2024. PMID: 38612930 Free PMC article.
References
-
- Moscatelli N., Lunetti P., Braccia C., Armirotti A., Pisanello F., De Vittorio M., Zara V., Ferramosca A. Comparative Proteomic Analysis of Proteins Involved in Bioenergetics Pathways Associated with Human Sperm Motility. Int. J. Mol. Sci. 2019;20:3000. doi: 10.3390/ijms20123000. - DOI - PMC - PubMed
-
- Andras P., Yueming Q., Chohan K.R., Shirley C.R., Wendy A., Sanjay B., Middleton F.A., Conkrite K.L., Maureen B., Nick G. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl. Acad. Sci. USA. 2006;103:14813–14818. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

