Purification and Identification of a Novel Angiotensin Converting Enzyme Inhibitory Peptide from the Enzymatic Hydrolysate of Lepidotrigla microptera
- PMID: 35804705
- PMCID: PMC9265830
- DOI: 10.3390/foods11131889
Purification and Identification of a Novel Angiotensin Converting Enzyme Inhibitory Peptide from the Enzymatic Hydrolysate of Lepidotrigla microptera
Abstract
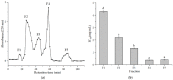
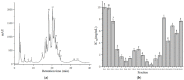
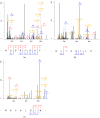
In this study, Lepidotrigla microptera were hydrolyzed with four different proteolytic enzymes (Papain, neutrase, flavourzyme, and alcalase), and their distribution of molecular weights and ACE-inhibitory activity were tested. The alcalase hydrolysates showed the maximum ACE-inhibitory activity. A novel ACE-inhibitory peptide was isolated and purified from Lepidotrigla microptera protein hydrolysate (LMPH) using ultrafiltration, gel filtration chromatography, and preparative high performance liquid chromatography (prep-HPLC). The amino acid sequence of the purified peptide was identified as Phe-Leu-Thr-Ala-Gly-Leu-Leu-Asp (DLTAGLLE), and the IC50 value was 0.13 mg/mL. The ACE-inhibitory activity of DLTAGLLE was stable across a range of temperatures (<100 °C) and pH values (3.0−11.0) and retained after gastrointestinal digestion. DLTAGLLE was further identified as a noncompetitive inhibitor by Lineweaver−Burk plot. The molecular docking simulation showed that DLTAGLLE showed a high binding affinity with ACE sites by seven short hydrogen bonds. As the first reported antihypertensive peptide extracted from alcalase hydrolysate of Lepidotrigla microptera, DLTAGLLE has the potential to develop functional food or novel ACE-inhibitor drugs.
Keywords: ACE inhibitory peptide; Lepidotrigla microptera; identification; molecular docking; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Purification and characterization of angiotensin I converting enzyme inhibitory peptides from the rotifer, Brachionus rotundiformis.Bioresour Technol. 2009 Nov;100(21):5255-9. doi: 10.1016/j.biortech.2009.05.057. Epub 2009 Jun 18. Bioresour Technol. 2009. PMID: 19540110
-
Purification, characterization and molecular docking study of angiotensin-I converting enzyme (ACE) inhibitory peptide from shortfin scad (Decapterus macrosoma) protein hydrolysate.J Food Sci Technol. 2021 Dec;58(12):4567-4577. doi: 10.1007/s13197-020-04944-y. Epub 2021 Jan 6. J Food Sci Technol. 2021. PMID: 34629521 Free PMC article.
-
Purification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from an enzymatic hydrolysate of duck skin byproducts.J Agric Food Chem. 2012 Oct 10;60(40):10035-40. doi: 10.1021/jf3023172. Epub 2012 Sep 28. J Agric Food Chem. 2012. PMID: 22994628
-
Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis.Mar Drugs. 2019 Mar 19;17(3):179. doi: 10.3390/md17030179. Mar Drugs. 2019. PMID: 30893907 Free PMC article.
-
Purification and the secondary structure of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the alcalase hydrolysate of seahorse protein.J Food Sci Technol. 2020 Nov;57(11):3927-3934. doi: 10.1007/s13197-020-04427-0. Epub 2020 Apr 15. J Food Sci Technol. 2020. PMID: 33071314 Free PMC article. Review.
Cited by
-
Biodirected Screening and Preparation of Larimichthys crocea Angiotensin-I-Converting Enzyme-Inhibitory Peptides by a Combined In Vitro and In Silico Approach.Molecules. 2024 Mar 3;29(5):1134. doi: 10.3390/molecules29051134. Molecules. 2024. PMID: 38474646 Free PMC article.
-
Preparation and Characterization of an Anticancer Peptide from Oriental Tonic Food Enteromorpha prolifera.Foods. 2022 Nov 4;11(21):3507. doi: 10.3390/foods11213507. Foods. 2022. PMID: 36360120 Free PMC article.
-
Purification, Identification and Molecular Docking of Immunomodulatory Peptides from the Heads of Litopenaeus vannamei.Foods. 2022 Oct 21;11(20):3309. doi: 10.3390/foods11203309. Foods. 2022. PMID: 37431056 Free PMC article.
-
From Sea to Lab: Angiotensin I-Converting Enzyme Inhibition by Marine Peptides-Mechanisms and Applications.Mar Drugs. 2024 Sep 30;22(10):449. doi: 10.3390/md22100449. Mar Drugs. 2024. PMID: 39452857 Free PMC article. Review.
-
Research Progress on the Mechanism of Action of Food-Derived ACE-Inhibitory Peptides.Life (Basel). 2025 Aug 1;15(8):1219. doi: 10.3390/life15081219. Life (Basel). 2025. PMID: 40868867 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

