Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet
- PMID: 35804799
- PMCID: PMC9265568
- DOI: 10.3390/foods11131984
Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet
Abstract
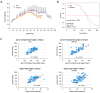
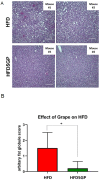
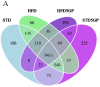
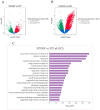
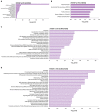
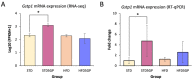
A key objective of this study was to explore the potential of dietary grape consumption to modulate adverse effects caused by a high-fat (western-pattern) diet. Female C57BL/6J mice were purchased at six-weeks-of-age and placed on a standard (semi-synthetic) diet (STD). At 11 weeks-of-age, the mice were continued on the STD or placed on the STD supplemented with 5% standardized grape powder (STD5GP), a high-fat diet (HFD), or an HFD supplemented with 5% standardized grape powder (HFD5GP). After being provided with the respective diets for 13 additional weeks, the mice were euthanized, and liver was collected for biomarker analysis, determination of genetic expression (RNA-Seq), and histopathological examination. All four dietary groups demonstrated unique genetic expression patterns. Using pathway analysis tools (GO, KEGG and Reactome), relative to the STD group, differentially expressed genes of the STD5GP group were significantly enriched in RNA, mitochondria, and protein translation related pathways, as well as drug metabolism, glutathione, detoxification, and oxidative stress associated pathways. The expression of Gstp1 was confirmed to be upregulated by about five-fold (RT-qPCR), and, based on RNA-Seq data, the expression of additional genes associated with the reduction of oxidative stress and detoxification (Gpx4 and 8, Gss, Gpx7, Sod1) were enhanced by dietary grape supplementation. Cluster analysis of genetic expression patterns revealed the greatest divergence between the HFD5GP and HFD groups. In the HFD5GP group, relative to the HFD group, 14 genes responsible for the metabolism, transportation, hydrolysis, and sequestration of fatty acids were upregulated. Conversely, genes responsible for lipid content and cholesterol synthesis (Plin4, Acaa1b, Slc27a1) were downregulated. The two top classifications emerging as enriched in the HFD5GP group vs. the HFD group (KEGG pathway analysis) were Alzheimer's disease and nonalcoholic fatty liver disease (NAFLD), both of which have been reported in the literature to bear a causal relationship. In the current study, nonalcoholic steatohepatitis was indicated by histological observations that revealed archetype markers of fatty liver induced by the HFD. The adverse response was diminished by grape intervention. In addition to these studies, life-long survival was assessed with C57BL/6J mice. C57BL/6J mice were received at four-weeks-of-age and placed on the STD. At 14-weeks-of-age, the mice were divided into two groups (100 per group) and provided with the HFD or the HFD5GP. Relative to the HFD group, the survival time of the HFD5GP group was enhanced (log-rank test, p = 0.036). The respective hazard ratios were 0.715 (HFD5GP) and 1.397 (HFD). Greater body weight positively correlated with longevity; the highest body weight of the HFD5GP group was attained later in life than the HFD group (p = 0.141). These results suggest the potential of dietary grapes to modulate hepatic gene expression, prevent oxidative damage, induce fatty acid metabolism, ameliorate NAFLD, and increase longevity when co-administered with a high-fat diet.
Keywords: C57BL/6J mice; RNA sequencing; grapes; high-fat diet; metabolic diseases; nutrigenomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Doll R. An epidemiological perspective of the biology of cancer. Cancer Res. 1978;38:3573–3583. - PubMed
-
- Phillips R.L. Role of life-style and dietary habits in risk of cancer among Seventh-day Adventists. Cancer Res. 1975;35:3513–3522. - PubMed
-
- Healthy Diet. [(accessed on 14 January 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

