Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies
- PMID: 35804818
- PMCID: PMC9264814
- DOI: 10.3390/cancers14133047
Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies
Abstract
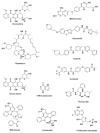
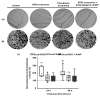
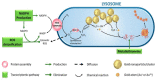
Hematological malignancies (HMs) are a heterogeneous group of blood neoplasia generally characterized by abnormal blood-cell production. Detection of HMs-specific molecular biomarkers (e.g., surface antigens, nucleic acid, and proteomic biomarkers) is crucial in determining clinical states and monitoring disease progression. Early diagnosis of HMs, followed by an effective treatment, can remarkably extend overall survival of patients. However, traditional and advanced HMs' diagnostic strategies still lack selectivity and sensitivity. More importantly, commercially available chemotherapeutic drugs are losing their efficacy due to adverse effects, and many patients develop resistance against these drugs. To overcome these limitations, the development of novel potent and reliable theranostic agents is urgently needed to diagnose and combat HMs at an early stage. Recently, gold nanomaterials (GNMs) have shown promise in the diagnosis and treatment of HMs. Magnetic resonance and the surface-plasmon-resonance properties of GNMs have made them a suitable candidate in the diagnosis of HMs via magnetic-resonance imaging and colorimetric or electrochemical sensing of cancer-specific biomarkers. Furthermore, GNMs-based photodynamic therapy, photothermal therapy, radiation therapy, and targeted drug delivery enhanced the selectivity and efficacy of anticancer drugs or drug candidates. Therefore, surface-tuned GNMs could be used as sensitive, reliable, and accurate early HMs, metastatic HMs, and MRD-detection tools, as well as selective, potent anticancer agents. However, GNMs may induce endothelial leakage to exacerbate cancer metastasis. Studies using clinical patient samples, patient-derived HMs models, or healthy-animal models could give a precise idea about their theranostic potential as well as biocompatibility. The present review will investigate the theranostic potential of vectorized GNMs in HMs and future challenges before clinical theranostic applications in HMs.
Keywords: diagnosis; gold nanomaterials; hematological malignancies; promise and challenges; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lapotko D., Lukianova E., Shnip A., Zheltov G., Potapnev M., Savitsky V., Klimovich O., Oraevsky A. Laser activated nanothermolysis of leukemia cells monitored by photothermal microscopy; Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2005: The Sixth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-Optics; San Jose, CA, USA. 25 April 2005; pp. 82–89.
Publication types
LinkOut - more resources
Full Text Sources

