Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells
- PMID: 35804883
- PMCID: PMC9265127
- DOI: 10.3390/cancers14133111
Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells
Abstract
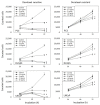
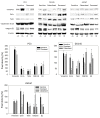
Despite recent advances in the treatment of metastatic prostate cancer (PCa), resistance development after taxane treatments is inevitable, necessitating effective options to combat drug resistance. Previous studies indicated antitumoral properties of the natural compound amygdalin. However, whether amygdalin acts on drug-resistant tumor cells remains questionable. An in vitro study was performed to investigate the influence of amygdalin (10 mg/mL) on the growth of a panel of therapy-naïve and docetaxel- or cabazitaxel-resistant PCa cell lines (PC3, DU145, and LNCaP cells). Tumor growth, proliferation, clonal growth, and cell cycle progression were investigated. The cell cycle regulating proteins (phospho)cdk1, (phospho)cdk2, cyclin A, cyclin B, p21, and p27 and the mammalian target of rapamycin (mTOR) pathway proteins (phospho)Akt, (phospho)Raptor, and (phospho)Rictor as well as integrin β1 and the cytoskeletal proteins vimentin, ezrin, talin, and cytokeratin 8/18 were assessed. Furthermore, chemotactic activity and adhesion to extracellular matrix components were analyzed. Amygdalin dose-dependently inhibited tumor growth and reduced tumor clones in all (parental and resistant) PCa cell lines, accompanied by a G0/G1 phase accumulation. Cell cycle regulating proteins were significantly altered by amygdalin. A moderate influence of amygdalin on tumor cell adhesion and chemotaxis was observed as well, paralleled by modifications of cytoskeletal proteins and the integrin β1 expression level. Amygdalin may, therefore, block tumor growth and disseminative characteristics of taxane-resistant PCa cells. Further studies are warranted to determine amygdalin's value as an antitumor drug.
Keywords: amygdalin; cabazitaxel; cabozantinib; complementary/alternative medicine (CAM); docetaxel; prostate cancer; resistant cell lines.
Conflict of interest statement
I.T.: Advisory Board: Sanofi, Pfizer, and MSD; Lecturer: Sanofi, Janssen, and Astellas; Congress travel support: Janssen, Astellas, Ipsen, and Pfizer.
Figures







References
-
- Global Burden of Disease Cancer C., Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. - DOI - PMC - PubMed
-
- Marchioni M., Di Nicola M., Primiceri G., Novara G., Castellan P., Paul A.K., Veccia A., Autorino R., Cindolo L., Schips L. New Antiandrogen Compounds Compared to Docetaxel for Metastatic Hormone Sensitive Prostate Cancer: Results from a Network Meta-Analysis. J. Urol. 2020;203:751–759. doi: 10.1097/JU.0000000000000636. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

