RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era
- PMID: 35804899
- PMCID: PMC9265130
- DOI: 10.3390/cancers14133127
RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era
Abstract
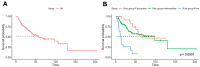
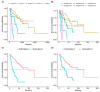
Immunotherapy has transformed the landscape of treatment in metastatic renal cell carcinoma (mRCC) in the last decade. Currently, prognostic risk stratification is based on the model developed in the era of vascular endothelial growth factor receptor inhibitors (VEGFRi) by Heng in 2009. Our study aims to find the most relevant risk criteria for mRCC patients treated with checkpoint inhibitors (CPI). In a retrospective cohort study, laboratory, pathology, demographic, and clinical data were retrieved from electronic medical records of consecutive mRCC patients treated with CPI in a tertiary center between 2015 and 2020. An unbiased multivariate analysis was performed to define predictive variables with a bootstrap validation step. We analyzed data on 127 patients with a median follow-up of 60 months. The median overall survival (OS) since the diagnosis of metastatic disease was 57 months. The response rate for CPI was 39%. Five risk factors were correlated with worse OS: intact primary kidney tumor (HR 2.33, p = 0.012), liver metastasis (HR 3.33, p = 0.001), <one year to treatment start (HR 1.98, p = 0.029), elevated platelets (HR 3.06, p = 0.015), and Karnofsky performance status <80% (HR = 3.42, p = 0.001). The model received a C-index of 70.7 compared with a score of 62.0 for the Heng’s model. When dividing patients into “low-risk” (0−1 risk factors) and “high-risk” (2−5 risk factors), there was good separation between the groups, with an HR of 5.9 (p < 0.0001). This study presents a new prognostic model for mRCC in the immunotherapy era with improved accuracy. Further research is needed to validate this model in larger cohorts.
Keywords: checkpoint inhibitors; renal cell carcinoma; risk prognostication.
Conflict of interest statement
Shira Sagie declares no conflict of interest. Michal Sarfaty reports honoraria from BMS, Pfizer, Janssen, and MSD. Meital Levartovsky reports honoraria from MSD, BMS, Medison, Astellas, and Roche and advisory from Novartis. Hadas Gantz Sorotsky reports honoraria from Roche-Genentech, MSD, AstraZeneca, Takeda, Astella, and BMS. Raanan Berger reports honoraria from BMS, MSD, Astellas, Pfizer, Medison, Roche, Janssen, and AstraZeneca and advisory from BMS, MSD, Astellas, Pfizer, Medison, Roche, Janssen, and AstraZeneca. Ruth Percik reports honoraria from BMS, MSD, and Novartis and an advisory board of Novartis and Janssen. Moran Gadot reports honoraria from MSD, Medison, BMS, Novartis, Astellas, Janssen, AstraZeneca, Pfizer, and Bayer. The authors declare no conflict of interest.
Figures
References
-
- Yang J.C., Sherry R.M., Steinberg S.M., Topalian S.L., Schwartzentruber D.J., Hwu P., Seipp C.A., Rogers-Freezer L., Morton K.E., White D.E., et al. Randomized Study of High—Dose and Low—Dose Inter leukin—2 in Patients with Metastatic Renal Cancer. J. Clin. Oncol. 2003;21:3127. doi: 10.1200/JCO.2003.02.122. - DOI - PMC - PubMed
-
- McDermott D.F., Regan M.M., Clark J.I., Flaherty L.E., Weiss G.R., Logan T.F., Kirkwood J.M., Gordon M.S., Sosman J.A., Ernstoff M.S., et al. Randomized Phase III Trial of High-Dose Interleukin-2 Versus Subcutaneous Interleukin-2 and Interferon in Patients With Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. - DOI - PubMed
LinkOut - more resources
Full Text Sources

