Dynamic Interactions between Tumor Cells and Brain Microvascular Endothelial Cells in Glioblastoma
- PMID: 35804908
- PMCID: PMC9265028
- DOI: 10.3390/cancers14133128
Dynamic Interactions between Tumor Cells and Brain Microvascular Endothelial Cells in Glioblastoma
Abstract
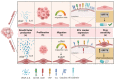
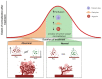
GBM is the most aggressive brain tumor among adults. It is characterized by extensive vascularization, and its further growth and recurrence depend on the formation of new blood vessels. In GBM, tumor angiogenesis is a multi-step process involving the proliferation, migration and differentiation of BMECs under the stimulation of specific signals derived from the cancer cells through a wide variety of communication routes. In this review, we discuss the dynamic interaction between BMECs and tumor cells by providing evidence of how tumor cells hijack the BMECs for the formation of new vessels. Tumor cell-BMECs interplay involves multiple routes of communication, including soluble factors, such as chemokines and cytokines, direct cell-cell contact and extracellular vesicles that participate in and fuel this cooperation. We also describe how this interaction is able to modify the BMECs structure, metabolism and physiology in a way that favors tumor growth and invasiveness. Finally, we briefly reviewed the recent advances and the potential future implications of some high-throughput 3D models to better understanding the complexity of BMECs-tumor cell interaction.
Keywords: angiogenesis; cancer; endothelial cells; extracellular vesicles; miRNA; neovascularization; tumor vessels normalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

