Cancer-Associated Fibroblasts Promote Tumor Aggressiveness in Head and Neck Cancer through Chemokine Ligand 11 and C-C Motif Chemokine Receptor 3 Signaling Circuit
- PMID: 35804913
- PMCID: PMC9264987
- DOI: 10.3390/cancers14133141
Cancer-Associated Fibroblasts Promote Tumor Aggressiveness in Head and Neck Cancer through Chemokine Ligand 11 and C-C Motif Chemokine Receptor 3 Signaling Circuit
Abstract
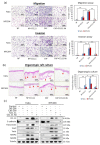
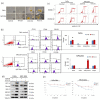
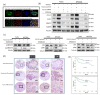
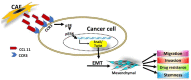
The tumor microenvironment (TME) plays a crucial role in tumor progression. One of its key stromal components, cancer-associated fibroblasts (CAFs), may crosstalk with cancer cells by secreting certain cytokines or chemokines. However, which important mediator(s) are released by CAFs, and the underlying molecular mechanism, remain largely unknown. In the present study, we isolated patient-derived CAFs and normal fibroblasts (NFs). Using microarray analysis, we detected chemokine ligand 11 (CCL11) overexpression in CAFs compared to NFs. CCL11 administration promoted the migration and invasion of head and neck cancer (HNC) cells with enhanced cancer stem cell-like properties and induction of epithelial-to-mesenchymal transition. Furthermore, neutralization of CCL11 activity reversed the aggressive phenotype of CAF-induced cancer cells. Confocal microscopy showed colocalization of CCL11 and CC chemokine receptor 3 (CCR3) on HNC cells. Moreover, immunohistochemical analysis of clinical samples from 104 patients with HNC showed that expression of CCL11 and CCR3 were significantly correlated with poor overall survival (p = 0.003 and 0.044, respectively). Collectively, CCL11 expressed on CAFs promotes HNC invasiveness, and neutralization of CCL11 reverses this effect. We propose that the CCL11/CCR3 signaling circuit is a potential target for optimizing therapeutic strategies against HNC.
Keywords: CCL11; CCR3; cancer-associated fibroblasts; head and neck cancer; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

