p53 Isoforms as Cancer Biomarkers and Therapeutic Targets
- PMID: 35804915
- PMCID: PMC9264937
- DOI: 10.3390/cancers14133145
p53 Isoforms as Cancer Biomarkers and Therapeutic Targets
Abstract
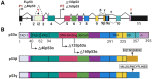
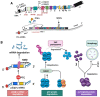
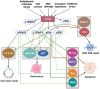
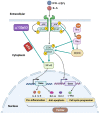
This review aims to summarize the implications of the major isoforms of the tumor suppressor protein p53 in aggressive cancer development. The current knowledge of p53 isoforms, their involvement in cell-signaling pathways, and their interactions with other cellular proteins or factors suggests the existence of an intricate molecular network that regulates their oncogenic function. Moreover, existing literature about the involvement of the p53 isoforms in various cancers leads to the proposition of therapeutic solutions by altering the cellular levels of the p53 isoforms. This review thus summarizes how the major p53 isoforms Δ40p53α/β/γ, Δ133p53α/β/γ, and Δ160p53α/β/γ might have clinical relevance in the diagnosis and effective treatments of cancer.
Keywords: biomarker; cancer; p53 isoforms; therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interaction of p53 with the Δ133p53α and Δ160p53α isoforms regulates p53 conformation and transcriptional activity.Cell Death Dis. 2024 Nov 19;15(11):845. doi: 10.1038/s41419-024-07213-4. Cell Death Dis. 2024. PMID: 39562560 Free PMC article.
-
Differential effects of diverse p53 isoforms on TAp73 transcriptional activity and apoptosis.Carcinogenesis. 2013 Mar;34(3):522-9. doi: 10.1093/carcin/bgs370. Epub 2012 Nov 27. Carcinogenesis. 2013. PMID: 23188674
-
The Δ133p53 Isoforms, Tuners of the p53 Pathway.Cancers (Basel). 2020 Nov 18;12(11):3422. doi: 10.3390/cancers12113422. Cancers (Basel). 2020. PMID: 33218139 Free PMC article. Review.
-
Δ122p53, a mouse model of Δ133p53α, enhances the tumor-suppressor activities of an attenuated p53 mutant.Cell Death Dis. 2015 Jun 11;6(6):e1783. doi: 10.1038/cddis.2015.149. Cell Death Dis. 2015. PMID: 26068791 Free PMC article.
-
p53 Isoforms in Cellular Senescence- and Ageing-Associated Biological and Physiological Functions.Int J Mol Sci. 2019 Nov 29;20(23):6023. doi: 10.3390/ijms20236023. Int J Mol Sci. 2019. PMID: 31795382 Free PMC article. Review.
Cited by
-
The recent advancements in the early detection of cancer biomarkers by DNAzyme-assisted aptasensors.J Nanobiotechnology. 2022 Oct 4;20(1):438. doi: 10.1186/s12951-022-01640-1. J Nanobiotechnology. 2022. PMID: 36195928 Free PMC article. Review.
-
The Important Role of Protein Kinases in the p53 Sestrin Signaling Pathway.Cancers (Basel). 2023 Nov 13;15(22):5390. doi: 10.3390/cancers15225390. Cancers (Basel). 2023. PMID: 38001650 Free PMC article. Review.
-
Toxocara Canis Increases the Potential of Breast Cancer by Reducing the Expression of the P53 Protein.Curr Mol Med. 2024;24(3):335-343. doi: 10.2174/1566524023666230320103506. Curr Mol Med. 2024. PMID: 36959144
-
Biomarkers for Early Cancer Detection: A Landscape View of Recent Advancements, Spotlighting Pancreatic and Liver Cancers.ACS Pharmacol Transl Sci. 2024 Feb 14;7(3):586-613. doi: 10.1021/acsptsci.3c00346. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481702 Free PMC article. Review.
-
p53: The Multifaceted Roles of Covalent Modifications in Cancer.Pharmaceuticals (Basel). 2024 Dec 13;17(12):1682. doi: 10.3390/ph17121682. Pharmaceuticals (Basel). 2024. PMID: 39770524 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

