Quantitative Analysis of the MGMT Methylation Status of Glioblastomas in Light of the 2021 WHO Classification
- PMID: 35804921
- PMCID: PMC9264886
- DOI: 10.3390/cancers14133149
Quantitative Analysis of the MGMT Methylation Status of Glioblastomas in Light of the 2021 WHO Classification
Abstract
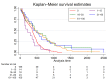
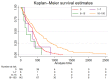
Background: Glioblastomas with methylation of the promoter region of the O(6)-methylguanine-DNA methyltransferase (MGMT) gene exhibit increased sensitivity to alkylating chemotherapy. Quantitative assessment of the MGMT promoter methylation status might provide additional prognostic information. The aim of our study was to determine a quantitative methylation threshold for better survival among patients with glioblastomas. Methods: We included consecutive patients ≥18 years treated at our department between 11/2010 and 08/2018 for a glioblastoma, IDH wildtype, undergoing quantitative MGMT promoter methylation analysis. The primary endpoint was overall survival. Results: A total of 321 patients were included. Median overall survival was 12.6 months. Kaplan−Meier and adjusted Cox regression analysis showed better survival for the groups with 16−30%, 31−60%, and 61−100% methylation. In contrast, survival in the group with 1−15% methylation was similar to those with unmethylated promoter regions. A secondary analysis confirmed this threshold. Conclusions: Better survival is observed in patients with glioblastomas with ≥16% methylation of the MGMT promoter region than with <16% methylation. Survival with tumors with 1−15% methylation is similar to with unmethylated tumors. Above 16% methylation, we found no additional benefit with increasing methylation.
Keywords: O(6)-methylguanine-DNA methyltransferase; glioblastoma; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest relevant to the manuscript.
Figures
Similar articles
-
Predictive value of MGMT promoter methylation on the survival of TMZ treated IDH-mutant glioblastoma.Cancer Biol Med. 2021 Feb 15;18(1):272-282. doi: 10.20892/j.issn.2095-3941.2020.0179. Cancer Biol Med. 2021. PMID: 33628600 Free PMC article.
-
O6-methylguanine-DNA methyltransferase promoter methylation and isocitrate dehydrogenase mutation as prognostic factors in a cohort of Saudi patients with glioblastoma.Ann Saudi Med. 2019 Nov-Dec;39(6):410-416. doi: 10.5144/0256-4947.2019.410. Epub 2019 Dec 5. Ann Saudi Med. 2019. PMID: 31804140 Free PMC article.
-
Prognostic value of O 6-methylguanine-DNA methyltransferase methylation in isocitrate dehydrogenase mutant gliomas.Neurooncol Adv. 2022 Mar 1;4(1):vdac030. doi: 10.1093/noajnl/vdac030. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 35386566 Free PMC article.
-
Somatic mutations in glioblastoma are associated with methylguanine-DNA methyltransferase methylation.Oncol Lett. 2015 May;9(5):2063-2067. doi: 10.3892/ol.2015.2980. Epub 2015 Feb 20. Oncol Lett. 2015. PMID: 26137013 Free PMC article.
-
Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy.Br J Cancer. 2009 Jul 7;101(1):124-31. doi: 10.1038/sj.bjc.6605127. Epub 2009 Jun 16. Br J Cancer. 2009. PMID: 19536096 Free PMC article.
Cited by
-
MGMT Promoter Methylation: Prognostication beyond Treatment Response.J Pers Med. 2023 Jun 14;13(6):999. doi: 10.3390/jpm13060999. J Pers Med. 2023. PMID: 37373988 Free PMC article.
-
4-Methylumbelliferone enhances the effects of chemotherapy on both temozolomide-sensitive and resistant glioblastoma cells.Sci Rep. 2023 Jun 8;13(1):9356. doi: 10.1038/s41598-023-35045-3. Sci Rep. 2023. PMID: 37291120 Free PMC article.
-
Predictive modeling with linear machine learning can estimate glioblastoma survival in months based solely on MGMT-methylation status, age and sex.Acta Neurochir (Wien). 2025 Feb 24;167(1):52. doi: 10.1007/s00701-025-06441-7. Acta Neurochir (Wien). 2025. PMID: 39992425 Free PMC article.
-
ATRX, OLIG2, MGMT, and IDH2 in Glioblastoma: Essential Molecular Mechanisms and Therapeutic Significance.Medicina (Kaunas). 2025 Apr 10;61(4):697. doi: 10.3390/medicina61040697. Medicina (Kaunas). 2025. PMID: 40282990 Free PMC article.
References
-
- Herrlinger U., Schäfer N., Steinbach J.P., Weyerbrock A., Hau P., Goldbrunner R., Friedrich F., Rohde V., Ringel F., Schlegel U., et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine–DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016;34:1611–1619. doi: 10.1200/JCO.2015.63.4691. - DOI - PubMed
-
- Gilbert M.R., Wang M., Aldape K.D., Stupp R., Hegi M.E., Jaeckle K.A., Armstrong T.S., Wefel J.S., Won M., Blumenthal D.T., et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

