Ovarian Adnexal Reporting Data System (O-RADS) for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis
- PMID: 35804924
- PMCID: PMC9264796
- DOI: 10.3390/cancers14133151
Ovarian Adnexal Reporting Data System (O-RADS) for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis
Abstract
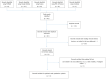
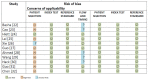
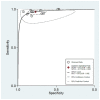
In this systematic review and meta-analysis, we aimed to assess the pooled diagnostic performance of the so-called Ovarian Adnexal Report Data System (O-RADS) for classifying adnexal masses using transvaginal ultrasound, a classification system that was introduced in 2020. We performed a search for studies reporting the use of the O-RADS system for classifying adnexal masses from January 2020 to April 2022 in several databases (Medline (PubMed), Google Scholar, Scopus, Cochrane, and Web of Science). We selected prospective and retrospective cohort studies using the O-RADS system for classifying adnexal masses with histologic diagnosis or conservative management demonstrating spontaneous resolution or persistence in cases of benign appearing masses after follow-up scan as the reference standard. We excluded studies not related to the topic under review, studies not addressing O-RADS classification, studies addressing MRI O-RADS classification, letters to the editor, commentaries, narrative reviews, consensus documents, and studies where data were not available for constructing a 2 × 2 table. The pooled sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratio (DOR) were calculated. The quality of the studies was evaluated using QUADAS-2. A total of 502 citations were identified. Ultimately, 11 studies comprising 4634 masses were included. The mean prevalence of ovarian malignancy was 32%. The risk of bias was high in eight studies for the "patient selection" domain. The risk of bias was low for the "index test" and "reference test" domains for all studies. Overall, the pooled estimated sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and DOR of the O-RADS system for classifying adnexal masses were 97% (95% confidence interval (CI) = 94%-98%), 77% (95% CI = 68%-84%), 4.2 (95% CI = 2.9-6.0), 0.04 (95% CI = 0.03-0.07), and 96 (95% CI = 50-185), respectively. Heterogeneity was moderate for sensitivity and high for specificity. In conclusion, the O-RADS system has good sensitivity and moderate specificity for classifying adnexal masses.
Keywords: O-RADS; benign neoplasms; malignancy; meta-analysis; ovarian cancer diagnosis; ovarian neoplasms; transvaginal ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Gynecologic Imaging and Reporting Data System for classifying adnexal masses.Minerva Obstet Gynecol. 2023 Feb;75(1):69-79. doi: 10.23736/S2724-606X.22.05122-3. Minerva Obstet Gynecol. 2023. PMID: 36790399
-
GI-RADS versus O-RADS in the differential diagnosis of adnexal masses: a systematic review and head-to-head meta-analysis.Ultrasonography. 2024 Nov;43(6):438-447. doi: 10.14366/usg.24105. Epub 2024 Sep 2. Ultrasonography. 2024. PMID: 39415417 Free PMC article.
-
Comparison of the Diagnostic Performance of Ovarian Adnexal Reporting Data System (O-RADS) With IOTA Simple Rules and ADNEX Model for Classifying Adnexal Masses: A Head-To-Head Meta-Analysis.J Clin Ultrasound. 2025 Apr 29. doi: 10.1002/jcu.24048. Online ahead of print. J Clin Ultrasound. 2025. PMID: 40298029 Review.
-
Diagnostic performance of the O-RADS MRI system for magnetic resonance imaging in discriminating benign and malignant adnexal lesions: a systematic review, meta-analysis, and meta-regression.Diagn Interv Radiol. 2025 Apr 28;31(3):171-179. doi: 10.4274/dir.2024.242784. Epub 2024 Jul 8. Diagn Interv Radiol. 2025. PMID: 38973658 Free PMC article.
-
Diagnostic performance of contrast-enhanced ultrasound (CEUS) combined with Ovarian-Adnexal Reporting and Data System (O-RADS) ultrasound risk stratification for adnexal masses: a systematic review and meta-analysis.Clin Radiol. 2024 Sep;79(9):e1167-e1175. doi: 10.1016/j.crad.2024.05.021. Epub 2024 Jun 7. Clin Radiol. 2024. PMID: 38942707
Cited by
-
Developing a deep learning model for predicting ovarian cancer in Ovarian-Adnexal Reporting and Data System Ultrasound (O-RADS US) Category 4 lesions: A multicenter study.J Cancer Res Clin Oncol. 2024 Jul 9;150(7):346. doi: 10.1007/s00432-024-05872-6. J Cancer Res Clin Oncol. 2024. PMID: 38981916 Free PMC article.
-
Effect of differences in O-RADS lexicon interpretation between senior and junior sonologists on O-RADS classification and diagnostic performance.J Cancer Res Clin Oncol. 2023 Oct;149(13):12275-12283. doi: 10.1007/s00432-023-05108-z. Epub 2023 Jul 11. J Cancer Res Clin Oncol. 2023. PMID: 37430161 Free PMC article.
-
Ovarian-Adnexal Imaging-Reporting and Data System (O-RADS) ultrasound version 2019: a prospective validation and comparison to updated version (v2022) in pathologically confirmed adnexal masses.Eur Radiol. 2025 Jun;35(6):3080-3095. doi: 10.1007/s00330-024-11235-z. Epub 2024 Nov 28. Eur Radiol. 2025. PMID: 39604652
-
A nomogram combining clinical features, O-RADS US, and radiomics based on ultrasound imaging for diagnosing ovarian cancer.Sci Rep. 2025 Jun 2;15(1):19279. doi: 10.1038/s41598-025-02776-4. Sci Rep. 2025. PMID: 40456815 Free PMC article.
-
Accurate prediction of benign and malignant adnexal tumors in surgical resection and conservative treatment: construction and external validation of a diagnostic model based on CEUS, HE4, and O-RADS US v2022 evaluation.J Ovarian Res. 2025 Jun 6;18(1):123. doi: 10.1186/s13048-025-01707-1. J Ovarian Res. 2025. PMID: 40481532 Free PMC article.
References
-
- Froyman W., Landolfo C., de Cock B., Wynants L., Sladkevicius P., Testa A.C., van Holsbeke C., Domali E., Fruscio R., Epstein E., et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019;20:448–458. doi: 10.1016/S1470-2045(18)30837-4. - DOI - PubMed
-
- The American College of Obstetricians and Gynecologists American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet. Gynecol. 2016;128:e210–e226. doi: 10.1097/AOG.0000000000001768. - DOI - PubMed
-
- Alcázar J.L., Aubá M., Ruiz-Zambrana A., Olartecoechea B., Diaz D., Hidalgo J.J., Pineda L., Utrilla-Layna J. Ultrasound assessment in adnexal masses: An update. Expert Rev. Obstet. Gynecol. 2012;7:441–449. doi: 10.1586/eog.12.49. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

