Preferential Antibody and Drug Conjugate Targeting of the ADAM10 Metalloprotease in Tumours
- PMID: 35804938
- PMCID: PMC9264901
- DOI: 10.3390/cancers14133171
Preferential Antibody and Drug Conjugate Targeting of the ADAM10 Metalloprotease in Tumours
Abstract
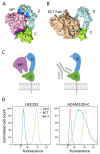
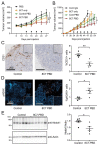
ADAM10 is a transmembrane metalloprotease that sheds a variety of cell surface proteins, including receptors and ligands that regulate a range of developmental processes which re-emerge during tumour development. While ADAM10 is ubiquitously expressed, its activity is normally tightly regulated, but becomes deregulated in tumours. We previously reported the generation of a monoclonal antibody, 8C7, which preferentially recognises an active form of ADAM10 in human and mouse tumours. We now report our investigation of the mechanism of this specificity, and the preferential targeting of 8C7 to human tumour cell xenografts in mice. We also report the development of novel 8C7 antibody-drug conjugates that preferentially kill cells displaying the 8C7 epitope, and that can inhibit tumour growth in mice. This study provides the first demonstration that antibody-drug conjugates targeting an active conformer of ADAM10, a widely expressed transmembrane metalloprotease, enable tumour-selective targeting and inhibition.
Keywords: ADAM metalloprotease; antibody–drug conjugate; brain cancer; colon cancer; therapeutic antibody.
Conflict of interest statement
P.W.J., A.M.S., N.S. and D.B.N. are inventors of granted patents relevant to the anti-ADAM10 antibody 8C7. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lubke T., Lena Illert A., von Figura K., et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

