Prospective Comparison of the Performance of MRI Versus CT in the Detection and Evaluation of Peritoneal Surface Malignancies
- PMID: 35804951
- PMCID: PMC9264985
- DOI: 10.3390/cancers14133179
Prospective Comparison of the Performance of MRI Versus CT in the Detection and Evaluation of Peritoneal Surface Malignancies
Abstract
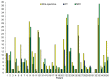
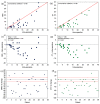
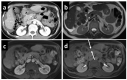
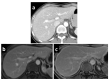
Background: The performance of MRI versus CT in the detection and evaluation of peritoneal surface malignancies (PSM) remains unclear in the current literature. Our study is the first prospective study in an Asian center comparing the two imaging modalities, validated against intra-operative findings. Methods: A total of 36 patients with PSM eligible for CRS-HIPEC underwent both MRI and CT scans up to 6 weeks before the operation. The scans were assessed for the presence and distribution of PSM and scored using the peritoneal cancer index (PCI), which were compared against PCI determined at surgery. Results: Both MRI and CT were 100% sensitive and specific in detecting the overall presence of PSM. Across all peritoneal regions, the sensitivity and specificity for PSM detection was 49.1% and 93.0% for MRI, compared to 47.8% and 95.1% for CT (p = 0.76). MRI was more sensitive than CT for small bowel disease, although the difference did not reach statistical significance. Comparing PCI on imaging with intra-operative PCI, the mean difference was found to be −3.4 ± 5.4 (p < 0.01) for MRI, and −3.9 ± 4.1 (p < 0.01) for CT. The correlation between imaging and intra-operative PCI was poor, with a concordance coefficient of 0.76 and 0.79 for MRI and CT, respectively. Within individual peritoneal regions, there was also poor agreement between imaging and intra-operative PCI for both modalities, other than in regions 1 and 3. Conclusion: MRI and CT are comparable in the detection and evaluation of PSM. While sensitive in the overall detection of PSM, they are likely to underestimate the true disease burden.
Keywords: computed tomography; cytoreductive surgery; intraperitoneal chemotherapy; magnetic resonance imaging; peritoneal cancer index; peritoneal metastases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Proposed radiological criteria for pre-operative determination of resectability in peritoneal-based malignancies.J Med Imaging Radiat Oncol. 2016 Jun;60(3):337-43. doi: 10.1111/1754-9485.12456. Epub 2016 Apr 21. J Med Imaging Radiat Oncol. 2016. PMID: 27098828
-
Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases.Cancer Imaging. 2019 Jan 7;19(1):1. doi: 10.1186/s40644-018-0187-z. Cancer Imaging. 2019. PMID: 30616608 Free PMC article.
-
Surgical Options for Peritoneal Surface Metastases from Digestive Malignancies-A Comprehensive Review.Medicina (Kaunas). 2023 Jan 28;59(2):255. doi: 10.3390/medicina59020255. Medicina (Kaunas). 2023. PMID: 36837456 Free PMC article. Review.
-
Predictive value of peritoneal cancer index for survival in patients with mucinous peritoneal malignancies treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a single centre experience.Int J Hyperthermia. 2018 Aug;34(5):512-517. doi: 10.1080/02656736.2017.1351627. Epub 2017 Jul 26. Int J Hyperthermia. 2018. PMID: 28679331
-
Diagnostic Laparoscopy in the Pre-operative Assessment of Patients Undergoing Cytoreductive Surgery and HIPEC for Peritoneal Surface Malignancies.Indian J Surg Oncol. 2016 Jun;7(2):230-5. doi: 10.1007/s13193-015-0486-9. Epub 2016 Jan 11. Indian J Surg Oncol. 2016. PMID: 27065714 Free PMC article. Review.
Cited by
-
Malignant mesothelioma tumours: molecular pathogenesis, diagnosis, and therapies accompanying clinical studies.Front Oncol. 2023 Jul 4;13:1204722. doi: 10.3389/fonc.2023.1204722. eCollection 2023. Front Oncol. 2023. PMID: 37469419 Free PMC article. Review.
-
CT-based radiomics nomogram for the pre-operative prediction of lymphovascular invasion in colorectal cancer: a multicenter study.Br J Radiol. 2023 Jan 1;96(1141):20220568. doi: 10.1259/bjr.20220568. Epub 2022 Nov 28. Br J Radiol. 2023. PMID: 36318241 Free PMC article.
-
Imaging Evaluation of Peritoneal Metastasis: Current and Promising Techniques.Korean J Radiol. 2024 Jan;25(1):86-102. doi: 10.3348/kjr.2023.0840. Korean J Radiol. 2024. PMID: 38184772 Free PMC article. Review.
-
A CT-Based Lung Radiomics Nomogram for Classifying the Severity of Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2024 Dec 11;19:2705-2717. doi: 10.2147/COPD.S483007. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39677830 Free PMC article.
-
What is the accuracy, sensitivity and specificity of the radiological peritoneal cancer index in repeat cytoreductive surgery: a retrospective study.World J Surg Oncol. 2025 Apr 11;23(1):138. doi: 10.1186/s12957-025-03775-5. World J Surg Oncol. 2025. PMID: 40217249 Free PMC article.
References
-
- Rajan F., Bhatt A. Evolving Role of CRS and HIPEC: Current Indications. In: Bhatt A., editor. Management of Peritoneal Metastases—Cytoreductive Surgery, HIPEC and Beyond. Springer; Singapore: 2018. pp. 3–14.
-
- Granieri S., Bonomi A., Frassini S., Chierici A.P., Bruno F., Paleino S., Kusamura S., Germini A., Facciorusso A., Deraco M., et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021;47:2757–2767. doi: 10.1016/j.ejso.2021.05.016. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

