Imaging of Pediatric Testicular and Para-Testicular Tumors: A Pictural Review
- PMID: 35804952
- PMCID: PMC9265135
- DOI: 10.3390/cancers14133180
Imaging of Pediatric Testicular and Para-Testicular Tumors: A Pictural Review
Abstract
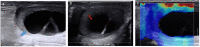
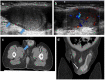
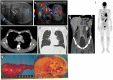
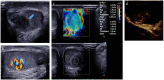
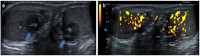
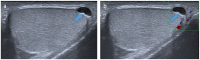
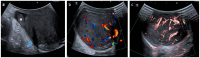
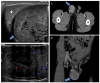
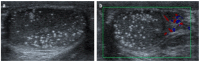
Pre- and post-pubertal testicular tumors are two distinct entities in terms of epidemiology, diagnosis and treatment. Most pre-pubertal tumors are benign; the most frequent are teratomas, and the most common malignant tumors are yolk-sac tumors. Post-pubertal tumors are similar to those found in adults and are more likely to be malignant. Imaging plays a pivotal role in the diagnosis, staging and follow-up. The appearance on ultrasonography (US) is especially helpful to differentiate benign lesions that could be candidates for testis-sparing surgery from malignant ones that require radical orchidectomy. Some specific imaging patterns are described for benign lesions: epidermoid cysts, mature cystic teratomas and Leydig-cell tumors. Benign tumors tend to be well-circumscribed, with decreased Doppler flow on US, but malignancy should be suspected when US shows an inhomogeneous, not-well-described lesion with internal blood flow. Imaging features should always be interpreted in combination with clinical and biological data including serum levels of tumor markers and even intra-operative frozen sections in case of conservative surgery to raise any concerns of malignity. This review provides an overview of imaging features of the most frequent testicular and para-testicular tumor types in children and the value of imaging in disease staging and monitoring children with testicular tumors or risk factors for testicular tumors.
Keywords: color Doppler; germ cell tumors; imaging; non-germ cell tumors; pediatric testicular tumors; pre-pubertal tumors; scrotal MRI; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

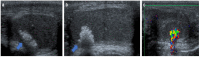
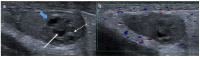
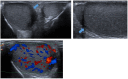
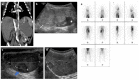

References
-
- Jiménez Isabel M.A., Gómez Fraile A., Aransay Brantot A., López Vázquez F., Delgado Muños M.D., Encinas Goenechea A., Matute de Cárdenas M.D., Berchi García F.J. Testicular tumors in childhood. Review of cases in the course of 13 years. Cir. Pediatr. 1996;9:13–16. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

