Testing for Dihydropyrimidine Dehydrogenase Deficiency to Individualize 5-Fluorouracil Therapy
- PMID: 35804978
- PMCID: PMC9264755
- DOI: 10.3390/cancers14133207
Testing for Dihydropyrimidine Dehydrogenase Deficiency to Individualize 5-Fluorouracil Therapy
Abstract
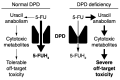
Severe adverse events (toxicity) related to the use of the commonly used chemotherapeutic drug 5-fluorouracil (5-FU) affect one in three patients and are the primary reason cited for premature discontinuation of therapy. Deficiency of the 5-FU catabolic enzyme dihydropyrimidine dehydrogenase (DPD, encoded by DPYD) has been recognized for the past 3 decades as a pharmacogenetic syndrome associated with high risk of 5-FU toxicity. An appreciable fraction of patients with DPD deficiency that receive 5-FU-based chemotherapy die as a result of toxicity. In this manuscript, we review recent progress in identifying actionable markers of DPD deficiency and the current status of integrating those markers into the clinical decision-making process. The limitations of currently available tests, as well as the regulatory status of pre-therapeutic DPYD testing, are also discussed.
Keywords: adverse events; chemotherapy; dihydropyrimidine dehydrogenase; fluorouracil; pharmacogenetics; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., Chiorean E.G., Chung V., Czito B., Del Chiaro M., et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. - DOI - PubMed
-
- Lee A.M., Shi Q., Pavey E., Alberts S.R., Sargent D.J., Sinicrope F.A., Berenberg J.L., Goldberg R.M., Diasio R.B. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147) J. Natl. Cancer Inst. 2014;106:dju298. doi: 10.1093/jnci/dju298. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous