Ca2+ Sensors Assemble: Function of the MCU Complex in the Pancreatic Beta Cell
- PMID: 35805078
- PMCID: PMC9265474
- DOI: 10.3390/cells11131993
Ca2+ Sensors Assemble: Function of the MCU Complex in the Pancreatic Beta Cell
Abstract
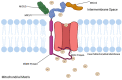
The Mitochondrial Calcium Uniporter Complex (MCU Complex) is essential for β-cell function due to its role in sustaining insulin secretion. The MCU complex regulates mitochondrial Ca2+ influx, which is necessary for increased ATP production following cellular glucose uptake, keeps the cell membrane K+ channels closed following initial insulin release, and ultimately results in sustained insulin granule exocytosis. Dysfunction in Ca2+ regulation results in an inability to sustain insulin secretion. This review defines the functions, structure, and mutations associated with the MCU complex members mitochondrial calcium uniporter protein (MCU), essential MCU regulator (EMRE), mitochondrial calcium uptake 1 (MICU1), mitochondrial calcium uptake 2 (MICU2), and mitochondrial calcium uptake 3 (MICU3) in the pancreatic β-cell. This review provides a framework for further evaluation of the MCU complex in β-cell function and insulin secretion.
Keywords: Ca2+ flux; EMRE; MCU complex; MICU1; MICU2; MICU3; pancreatic β-cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- McCulloch L.J., van de Bunt M., Braun M., Frayn K.N., Clark A., Gloyn A.L. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 2011;104:648–653. doi: 10.1016/j.ymgme.2011.08.026. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

