Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay
- PMID: 35805084
- PMCID: PMC9266202
- DOI: 10.3390/cells11132000
Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay
Abstract
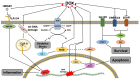
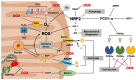
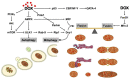
Cardiotoxicity has emerged as a major side effect of doxorubicin (DOX) treatment, affecting nearly 30% of patients within 5 years after chemotherapy. Heart failure is the first non-cancer cause of death in DOX-treated patients. Although many different molecular mechanisms explaining the cardiac derangements induced by DOX were identified in past decades, the translation to clinical practice has remained elusive to date. This review examines the current understanding of DOX-induced cardiomyopathy (DCM) with a focus on mitochondria, which were increasingly proven to be crucial determinants of DOX-induced cytotoxicity. We discuss DCM pathophysiology and epidemiology and DOX-induced detrimental effects on mitochondrial function, dynamics, biogenesis, and autophagy. Lastly, we review the current perspectives to contrast the development of DCM, which is still a relatively diffused, invalidating, and life-threatening condition for cancer survivors.
Keywords: DOX; anthracycline; cardiomyopathy; heart; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., Aboyans V., Asteggiano R., Galderisi M., Habib G., Lenihan D.J., Lip G.Y.H., Lyon A.R., et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

