Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives
- PMID: 35805142
- PMCID: PMC9265592
- DOI: 10.3390/cells11132058
Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives
Abstract
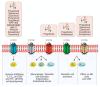
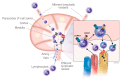
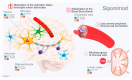
Sphingosine-1-phosphate (S1P) and S1P receptors (S1PR) are bioactive lipid molecules that are ubiquitously expressed in the human body and play an important role in the immune system. S1P-S1PR signaling has been well characterized in immune trafficking and activation in both innate and adaptive immune systems. Despite this knowledge, the full scope in the pathogenesis of autoimmune disorders is not well characterized yet. From the discovery of fingolimod, the first S1P modulator, until siponimod, the new molecule recently approved for the treatment of secondary progressive multiple sclerosis (SPMS), there has been a great advance in understanding the S1P functions and their involvement in immune diseases, including multiple sclerosis (MS). Modulation on S1P is an interesting target for the treatment of various autoimmune disorders. Improved understanding of the mechanism of action of fingolimod has allowed the development of the more selective second-generation S1PR modulators. Subtype 1 of the S1PR (S1PR1) is expressed on the cell surface of lymphocytes, which are known to play a major role in MS pathogenesis. The understanding of S1PR1's role facilitated the development of pharmacological strategies directed to this target, and theoretically reduced the safety concerns derived from the use of fingolimod. A great advance in the MS treatment was achieved in March 2019 when the Food and Drug Association (FDA) approved Siponimod, for both active secondary progressive MS and relapsing-remitting MS. Siponimod became the first oral disease modifying therapy (DMT) specifically approved for active forms of secondary progressive MS. Additionally, for the treatment of relapsing forms of MS, ozanimod was approved by FDA in March 2020. Currently, there are ongoing trials focused on other new-generation S1PR1 modulators. This review approaches the fundamental aspects of the sphingosine phosphate modulators and their main similarities and differences.
Keywords: S1P; S1PR1; fingolimod; multiple sclerosis; ozanimod; siponimod.
Conflict of interest statement
The authors declare no conflict of interest for the preparation of this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

