Identification and Characterization of a Novel Species of Genus Akkermansia with Metabolic Health Effects in a Diet-Induced Obesity Mouse Model
- PMID: 35805168
- PMCID: PMC9265676
- DOI: 10.3390/cells11132084
Identification and Characterization of a Novel Species of Genus Akkermansia with Metabolic Health Effects in a Diet-Induced Obesity Mouse Model
Abstract
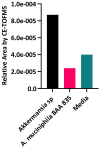
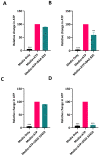
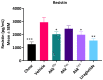
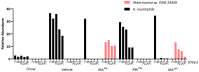
Akkermansia muciniphila is a well-known bacterium with the ability to degrade mucin. This metabolic capability is believed to play an important role in the colonization of this bacterium in the gut. In this study, we report the identification and characterization of a novel Akkermansia sp. DSM 33459 isolated from human feces of a healthy donor. Phylogenetic analysis based on the genome-wide average nucleotide identity indicated that the Akkermansia sp. DSM 33459 has only 87.5% similarity with the type strain A. muciniphila ATCC BAA-835. Akkermansia sp. DSM 33459 showed significant differences in its fatty acid profile and carbon utilization as compared to the type strain. The Akkermansia sp. DSM 33459 strain was tested in a preclinical obesity model to determine its effect on metabolic markers. Akkermansia sp. DSM 33459 showed significant improvement in body weight, total fat weight, and resistin and insulin levels. Interestingly, these effects were more pronounced with the live form as compared to a pasteurized form of the strain. The strain showed production of agmatine, suggesting a potential novel mechanism for supporting metabolic and cognitive health. Based on its phenotypic features and phylogenetic position, it is proposed that this isolate represents a novel species in the genus Akkermansia and a promising therapeutic candidate for the management of metabolic diseases.
Keywords: Akkermansia sp.; metabolic health; microbiome.
Conflict of interest statement
All authors were employed by IFF at the time the study was conducted and IFF provided the funding for the studies. R.K., H.K., Q.W., A.H., H.M.J., H.-S.K., P.R., A.C.O., S.D.F. and O.H. filed a patent for the use of
Figures







References
-
- Collado M.C., Derrien M., Isolauri E., de Vos W.M., Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

