Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF)
- PMID: 35805179
- PMCID: PMC9266271
- DOI: 10.3390/cells11132095
Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF)
Abstract
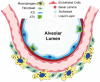
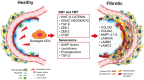
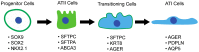
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease (ILD) with unknown etiology in which gradual fibrotic scarring of the lungs leads to usual interstitial pneumonia (UIP) and, ultimately, to death. IPF affects three million people worldwide, and the only currently available treatments include the antifibrotic drugs nintedanib and pirfenidone, which effectively reduce fibrosis progression are, unfortunately, not effective in curing the disease. In recent years, the paradigm of IPF pathogenesis has shifted from a fibroblast-driven disease to an epithelium-driven disease, wherein, upon recurrent microinjuries, dysfunctional alveolar type II epithelial cells (ATII) are not only unable to sustain physiological lung regeneration but also promote aberrant epithelial-mesenchymal crosstalk. This creates a drift towards fibrosis rather than regeneration. In the context of this review article, we discuss the most relevant mechanisms involved in IPF pathogenesis with a specific focus on the role of dysfunctional ATII cells in promoting disease progression. In particular, we summarize the main causes of ATII cell dysfunction, such as aging, environmental factors, and genetic determinants. Next, we describe the known mechanisms of physiological lung regeneration by drawing a parallel between embryonic lung development and the known pathways involved in ATII-driven alveolar re-epithelization after injury. Finally, we review the most relevant interventional clinical trials performed in the last 20 years with the aim of underlining the urgency of developing new therapies against IPF that are not only aimed at reducing disease progression by hampering ECM deposition but also boost the physiological processes of ATII-driven alveolar regeneration.
Keywords: ATII cells; IPF; regeneration; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

