Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility
- PMID: 35805890
- PMCID: PMC9266556
- DOI: 10.3390/ijms23136884
Enterohemorrhagic Escherichia coli and a Fresh View on Shiga Toxin-Binding Glycosphingolipids of Primary Human Kidney and Colon Epithelial Cells and Their Toxin Susceptibility
Abstract
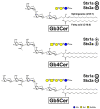
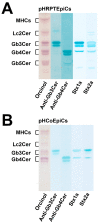
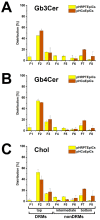
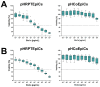
Enterohemorrhagic Escherichia coli (EHEC) are the human pathogenic subset of Shiga toxin (Stx)-producing E. coli (STEC). EHEC are responsible for severe colon infections associated with life-threatening extraintestinal complications such as the hemolytic-uremic syndrome (HUS) and neurological disturbances. Endothelial cells in various human organs are renowned targets of Stx, whereas the role of epithelial cells of colon and kidneys in the infection process has been and is still a matter of debate. This review shortly addresses the clinical impact of EHEC infections, novel aspects of vesicular package of Stx in the intestine and the blood stream as well as Stx-mediated extraintestinal complications and therapeutic options. Here follows a compilation of the Stx-binding glycosphingolipids (GSLs), globotriaosylceramide (Gb3Cer) and globotetraosylceramide (Gb4Cer) and their various lipoforms present in primary human kidney and colon epithelial cells and their distribution in lipid raft-analog membrane preparations. The last issues are the high and extremely low susceptibility of primary renal and colonic epithelial cells, respectively, suggesting a large resilience of the intestinal epithelium against the human-pathogenic Stx1a- and Stx2a-subtypes due to the low content of the high-affinity Stx-receptor Gb3Cer in colon epithelial cells. The review closes with a brief outlook on future challenges of Stx research.
Keywords: EHEC; STEC; Stx1a; Stx2a; detergent-resistant membranes; glycolipids; lipid rafts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
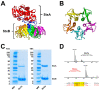
 ), weak binding to Gb4Cer (
), weak binding to Gb4Cer ( ), and no binding at all to Gb5Cer (
), and no binding at all to Gb5Cer ( ).
).

Similar articles
-
Primary Human Colon Epithelial Cells (pHCoEpiCs) Do Express the Shiga Toxin (Stx) Receptor Glycosphingolipids Gb3Cer and Gb4Cer and Are Largely Refractory but Not Resistant towards Stx.Int J Mol Sci. 2021 Sep 16;22(18):10002. doi: 10.3390/ijms221810002. Int J Mol Sci. 2021. PMID: 34576167 Free PMC article.
-
Shiga Toxin (Stx)-Binding Glycosphingolipids of Primary Human Renal Cortical Epithelial Cells (pHRCEpiCs) and Stx-Mediated Cytotoxicity.Toxins (Basel). 2021 Feb 12;13(2):139. doi: 10.3390/toxins13020139. Toxins (Basel). 2021. PMID: 33673393 Free PMC article.
-
Primary Human Renal Proximal Tubular Epithelial Cells (pHRPTEpiCs): Shiga Toxin (Stx) Glycosphingolipid Receptors, Stx Susceptibility, and Interaction with Membrane Microdomains.Toxins (Basel). 2021 Jul 28;13(8):529. doi: 10.3390/toxins13080529. Toxins (Basel). 2021. PMID: 34437399 Free PMC article.
-
Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells.Int J Med Microbiol. 2018 Dec;308(8):1073-1084. doi: 10.1016/j.ijmm.2018.09.003. Epub 2018 Sep 8. Int J Med Microbiol. 2018. PMID: 30224239 Review.
-
The Fatal Role of Enterohaemorrhagic Escherichia coli Shiga Toxin-associated Extracellular Vesicles in Host Cells.J Microbiol. 2023 Aug;61(8):715-727. doi: 10.1007/s12275-023-00066-0. Epub 2023 Sep 4. J Microbiol. 2023. PMID: 37665555 Review.
Cited by
-
Loop mediated isothermal amplification as a molecular diagnostic assay: Application and evaluation for detection of Enterohaemorrhagic Escherichia coli (O157:H7).Pract Lab Med. 2023 Sep 4;37:e00333. doi: 10.1016/j.plabm.2023.e00333. eCollection 2023 Nov. Pract Lab Med. 2023. PMID: 37693632 Free PMC article.
-
Shiga Toxin (Stx) Type 1a and Stx2a Translocate through a Three-Layer Intestinal Model.Toxins (Basel). 2023 Mar 9;15(3):207. doi: 10.3390/toxins15030207. Toxins (Basel). 2023. PMID: 36977098 Free PMC article.
-
Scientific Discoveries Supporting Theories in Science: From Thinking to Practice.Int J Mol Sci. 2022 Nov 30;23(23):15025. doi: 10.3390/ijms232315025. Int J Mol Sci. 2022. PMID: 36499354 Free PMC article.
-
A Mechanistic Approach to Replacing Antibiotics with Natural Products in the Treatment of Bacterial Diarrhea.Biomolecules. 2025 Jul 18;15(7):1045. doi: 10.3390/biom15071045. Biomolecules. 2025. PMID: 40723916 Free PMC article. Review.
-
Genomic island-encoded regulatory proteins in enterohemorrhagic Escherichia coli O157:H7.Virulence. 2024 Dec;15(1):2313407. doi: 10.1080/21505594.2024.2313407. Epub 2024 Feb 15. Virulence. 2024. PMID: 38357901 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

