1,3-Benzodioxole Derivatives Improve the Anti-Tumor Efficiency of Arsenicals
- PMID: 35805931
- PMCID: PMC9266561
- DOI: 10.3390/ijms23136930
1,3-Benzodioxole Derivatives Improve the Anti-Tumor Efficiency of Arsenicals
Abstract
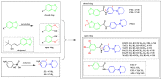
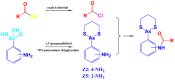
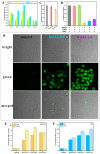
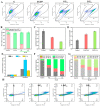
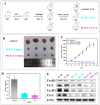
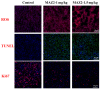
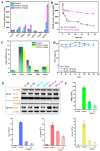
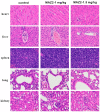
Arsenicals have been widely used in the treatment of cancers such as leukemia and other tumors. However, their side effects limit their clinical application. Stiripentol, a second-line adjunctive treatment for epilepsy with a good safety profile, inhibits microsomal cytochrome-P450-family enzymes to extend the retention time of co-administration. Inspired by the metabolism of stiripentol, the 1,3-benzodioxole responsible for the inhibition and its metabolic derivatives were conjugated with arsenical precursors. The fabricated arsenicals were eliminated much slower in mice and maintained an efficient concentration in the blood for a longer time than that of the arsenical precursors. They also performed better in anti-proliferation by inhibiting the thioredoxin system to induce oxidative stress, and concomitantly to initiate apoptosis in vitro and in vivo. The fabricated arsenicals reversed the hemogram of tumor-bearing mice to normal and eliminated the tumor without causing damage to any organs, exhibiting a good design strategy and pre-clinical application for leukemia and other tumors.
Keywords: 4T1 tumor; ROS; TrxR; docking; organic arsenicals; stiripentol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Organic arsenicals target thioredoxin reductase followed by oxidative stress and mitochondrial dysfunction resulting in apoptosis.Eur J Med Chem. 2018 Jan 1;143:1090-1102. doi: 10.1016/j.ejmech.2017.05.022. Epub 2017 May 6. Eur J Med Chem. 2018. PMID: 29150332
-
LDHA Suppression Altering Metabolism Inhibits Tumor Progress by an Organic Arsenical.Int J Mol Sci. 2019 Dec 11;20(24):6239. doi: 10.3390/ijms20246239. Int J Mol Sci. 2019. PMID: 31835667 Free PMC article.
-
Structural Modification of Aminophenylarsenoxides Generates Candidates for Leukemia Treatment via Thioredoxin Reductase Inhibition.J Med Chem. 2021 Nov 11;64(21):16132-16146. doi: 10.1021/acs.jmedchem.1c01441. Epub 2021 Oct 27. J Med Chem. 2021. PMID: 34704769
-
Darinaparsin: a novel organic arsenical with promising anticancer activity.Expert Opin Investig Drugs. 2009 Nov;18(11):1727-34. doi: 10.1517/13543780903282759. Expert Opin Investig Drugs. 2009. PMID: 19780704 Review.
-
[Advances in the study on antineoplastic action of realgar].Zhong Yao Cai. 2004 Mar;27(3):226-9. Zhong Yao Cai. 2004. PMID: 15346589 Review. Chinese. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

