Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series
- PMID: 35805941
- PMCID: PMC9266869
- DOI: 10.3390/ijms23136940
Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series
Abstract
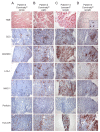
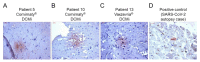
Myocarditis in response to COVID-19 vaccination has been reported since early 2021. In particular, young male individuals have been identified to exhibit an increased risk of myocardial inflammation following the administration of mRNA-based vaccines. Even though the first epidemiological analyses and numerous case reports investigated potential relationships, endomyocardial biopsy (EMB)-proven cases are limited. Here, we present a comprehensive histopathological analysis of EMBs from 15 patients with reduced ejection fraction (LVEF = 30 (14-39)%) and the clinical suspicion of myocarditis following vaccination with Comirnaty® (Pfizer-BioNTech) (n = 11), Vaxzevria® (AstraZenica) (n = 2) and Janssen® (Johnson & Johnson) (n = 2). Immunohistochemical EMB analyses reveal myocardial inflammation in 14 of 15 patients, with the histopathological diagnosis of active myocarditis according the Dallas criteria (n = 2), severe giant cell myocarditis (n = 2) and inflammatory cardiomyopathy (n = 10). Importantly, infectious causes have been excluded in all patients. The SARS-CoV-2 spike protein has been detected sparsely on cardiomyocytes of nine patients, and differential analysis of inflammatory markers such as CD4+ and CD8+ T cells suggests that the inflammatory response triggered by the vaccine may be of autoimmunological origin. Although a definitive causal relationship between COVID-19 vaccination and the occurrence of myocardial inflammation cannot be demonstrated in this study, data suggest a temporal connection. The expression of SARS-CoV-2 spike protein within the heart and the dominance of CD4+ lymphocytic infiltrates indicate an autoimmunological response to the vaccination.
Keywords: COVID-19; Comirnaty; Janssen; SARS-CoV-2; Vaxzevria; giant cell myocarditis; inflammatory cardiomyopathy; myocarditis; vaccination.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- CDC Centers for Disease Control and Prevention Myocarditis and Pericarditis Following MRNA COVID-19 Vaccination. [(accessed on 6 April 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html.
-
- Muthukumar A., Narasimhan M., Li Q.-Z., Mahimainathan L., Hitto I., Fuda F., Batra K., Jiang X., Zhu C., Schoggins J., et al. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 MRNA Vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

