Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response
- PMID: 35805965
- PMCID: PMC9266456
- DOI: 10.3390/ijms23136966
Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response
Abstract
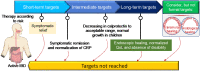
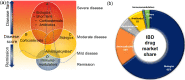
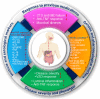
Inflammatory bowel disease (IBD) is a chronic immune-mediated inflammation of the gastrointestinal tract with a highly heterogeneous presentation. It has a relapsing and remitting clinical course that necessitates lifelong monitoring and treatment. Although the availability of a variety of effective therapeutic options including immunomodulators and biologics (such as TNF, CAM inhibitors) has led to a paradigm shift in the treatment outcomes and clinical management of IBD patients, some patients still either fail to respond or lose their responsiveness to therapy over time. Therefore, according to the recent Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) recommendations, continuous disease monitoring from symptomatic relief to endoscopic healing along with short- and long-term therapeutic responses are critical for providing IBD patients with a tailored therapy algorithm. Moreover, considering the high unmet need for novel therapeutic approaches for IBD patients, various new modulators of cytokine signaling events (for example, JAK/TYK inhibitors), inhibitors of cytokines (for example IL-12/IL-23, IL-22, IL-36, and IL-6 inhibitors), anti-adhesion and migration strategies (for example, β7 integrin, sphingosine 1-phosphate receptors, and stem cells), as well as microbial-based therapeutics to decolonize the bed buds (for example, fecal microbiota transplantation and bacterial inhibitors) are currently being evaluated in different phases of controlled clinical trials. This review aims to offer a comprehensive overview of available treatment options and emerging therapeutic approaches for IBD patients. Furthermore, predictive biomarkers for monitoring the therapeutic response to different IBD therapies are also discussed.
Keywords: Crohn’s disease; IBD; biological treatment; biomarkers; precision medicine; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Verstockt B., Bressler B., Martinez-Lozano H., McGovern D., Silverberg M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology. 2022;162:1370–1382. doi: 10.1053/j.gastro.2021.12.246. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

